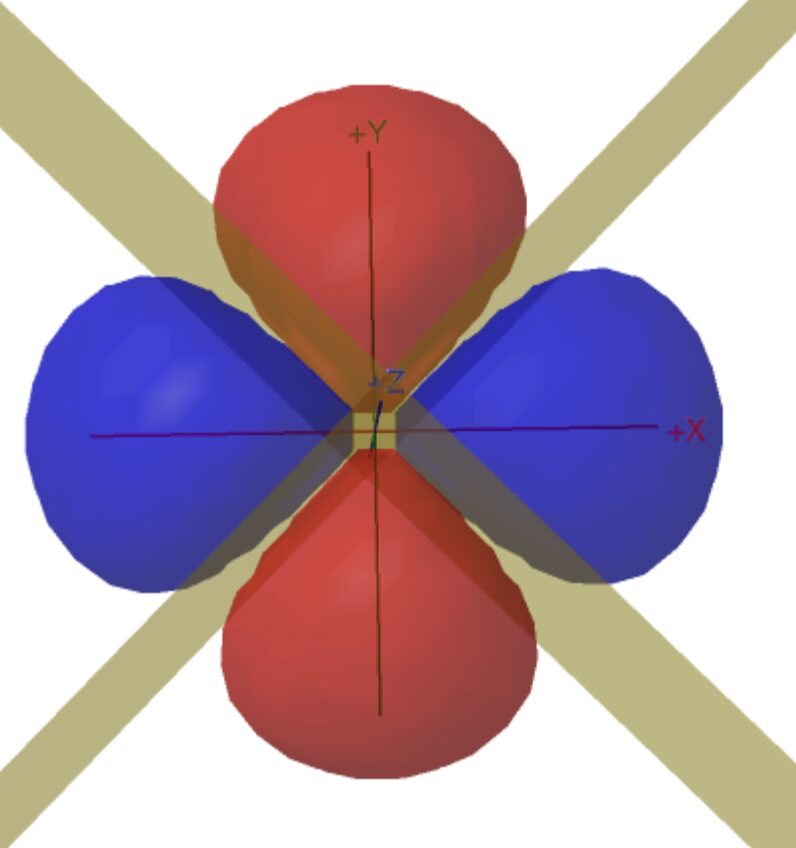
The pages displaying atomic orbitals in 3D (s,p,d,f) have always been very popular. This is because it can be difficult to imagine what these complex surfaces look like. Different pages display s-orbitals (1s, 2s, 3s), p-orbitals (2p and 3p), and the most popular is 3d-orbitals. f–orbitals are also available for those interested in the lanthanides.
You can also compare the relative sizes of 1s, 2s, 3s, and 2p orbitals.
This is useful for A level students and above.
We have recently added slices through the electron density to make radial nodes visible and planar and conical nodes are now optional for 2p, 3p, and 3d orbitals. The energy of the orbital increases with the number of nodes so visualising them also helps understands the relative energy of the various orbitals.