Click the structures and reaction arrows in sequence to view the 3D models and animations respectively
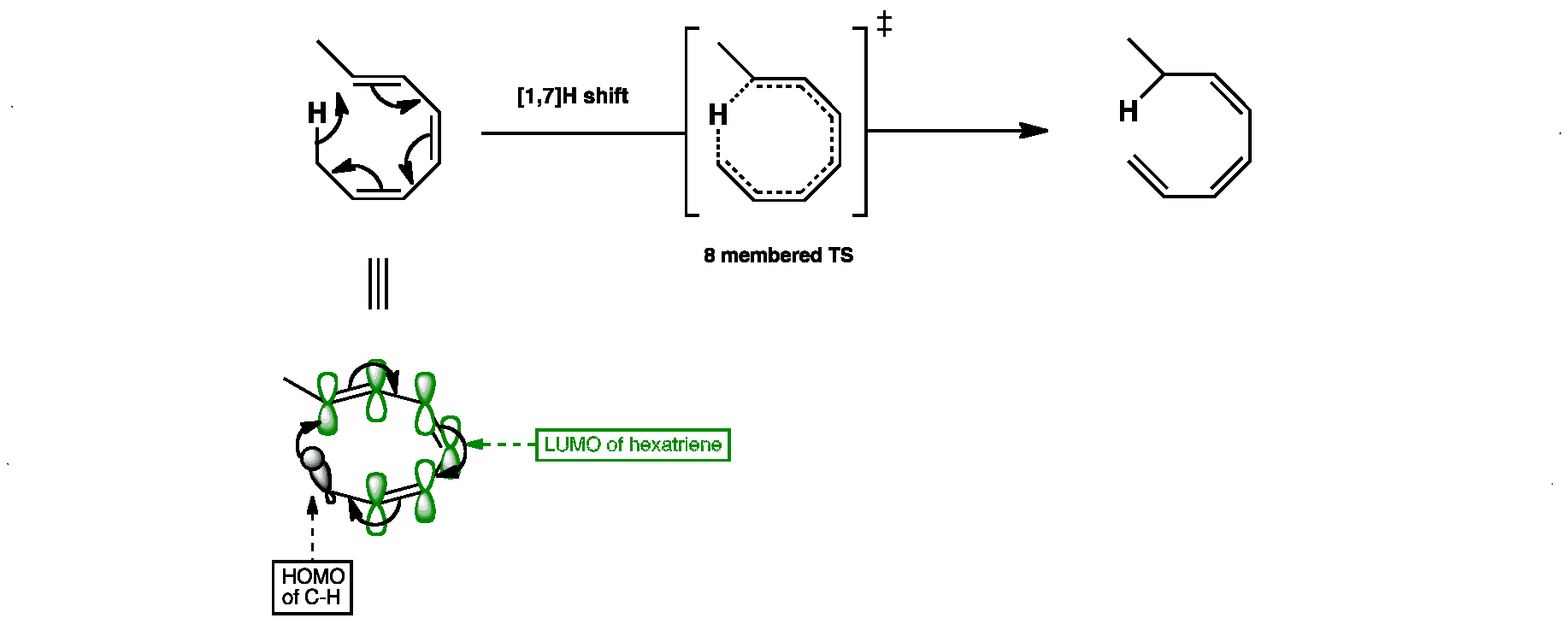
In thermal reactions, [1,5]H shifts occur suprafacially but [1,3]H and [1,7]H shifts must be antarafacial. Antarafacial [1,3]H shifts are impossible because a rigid 3-carbon chain is too short to allow the H atom to transfer from the top to the bottom. For [1,7]H shifts the much longer chain is flexible enough to allow the transfer.
The hydrogen atom leaves the top side of the triene and adds back in on the bottom side.
J. Clarke, P. W. Fowler, S. Gronert and J. R. Keeffe, J. Org. Chem., 2016, 81, 8777–8788.