Use the buttons to display the dipoles, charges and electrostatic potential mapped onto the surface of each molecule. Red regions are nucleophilic (electron rich) and blue regions are electrophilic (electron poor)
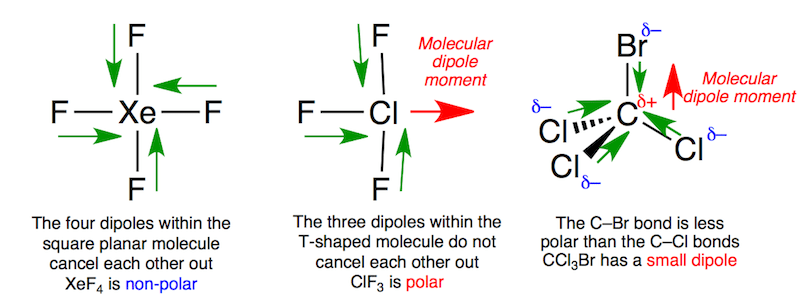
Xenon tetrafluoride is square planar. The Xe-F bonds are all polarized but they cancel one another out so the molecule has no dipole. Chlorine trifluoride has three polarized bonds and they combine to produce a small molecular dipole along the Cl-F bond.
Bromotrichloromethane is tetrahedral. The less polar C-Br bond does not quite balance the more polar C-Cl bonds so there is a small molecular dipole.
More molecules: Small Polar | Conjugated | Lone Pair Conjugation | Carbonyl Hydration