Click the structures and reaction arrows in sequence to view the 3D models and animations respectively
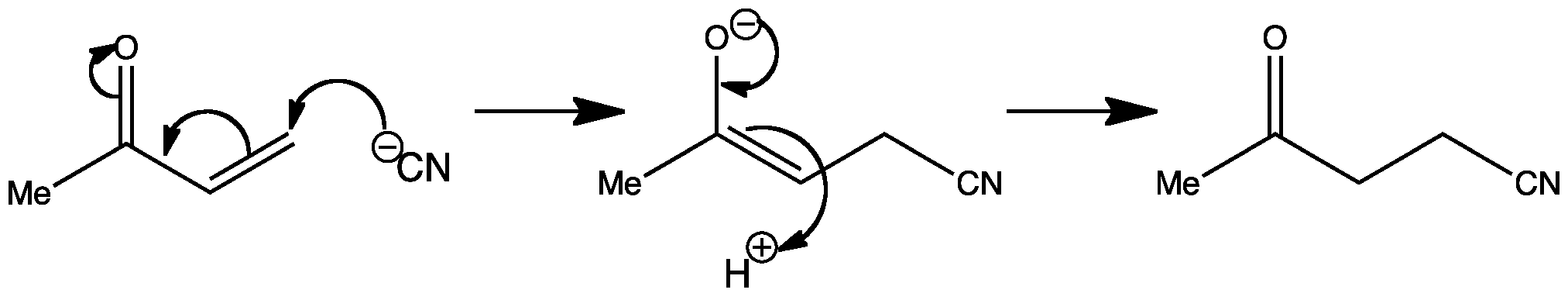
Small differences in reaction conditions, (such as temperature) can completely change the outcome of a reaction. In the first reaction shown below, you can see that it is an example of nucleophilic addition of cyanide to a ketone, giving a cyanohydrin:
Click the image to view cyanohydrin formation
However, when the temperature of the reaction is increased to 80°C, conjugate addition occurs, resulting in the addition of cyanide to the C=C double bond. Compounds with double bonds adjacent to a C=O group are known as α,β-unsaturated carbonyl compounds.
A. G. Csákÿ, G. de la Herrán and M. C. Murcia, Chem. Soc. Rev., 2010, 39, 4080.