Click the structures and reaction arrows in sequence to view the 3D models and animations respectively
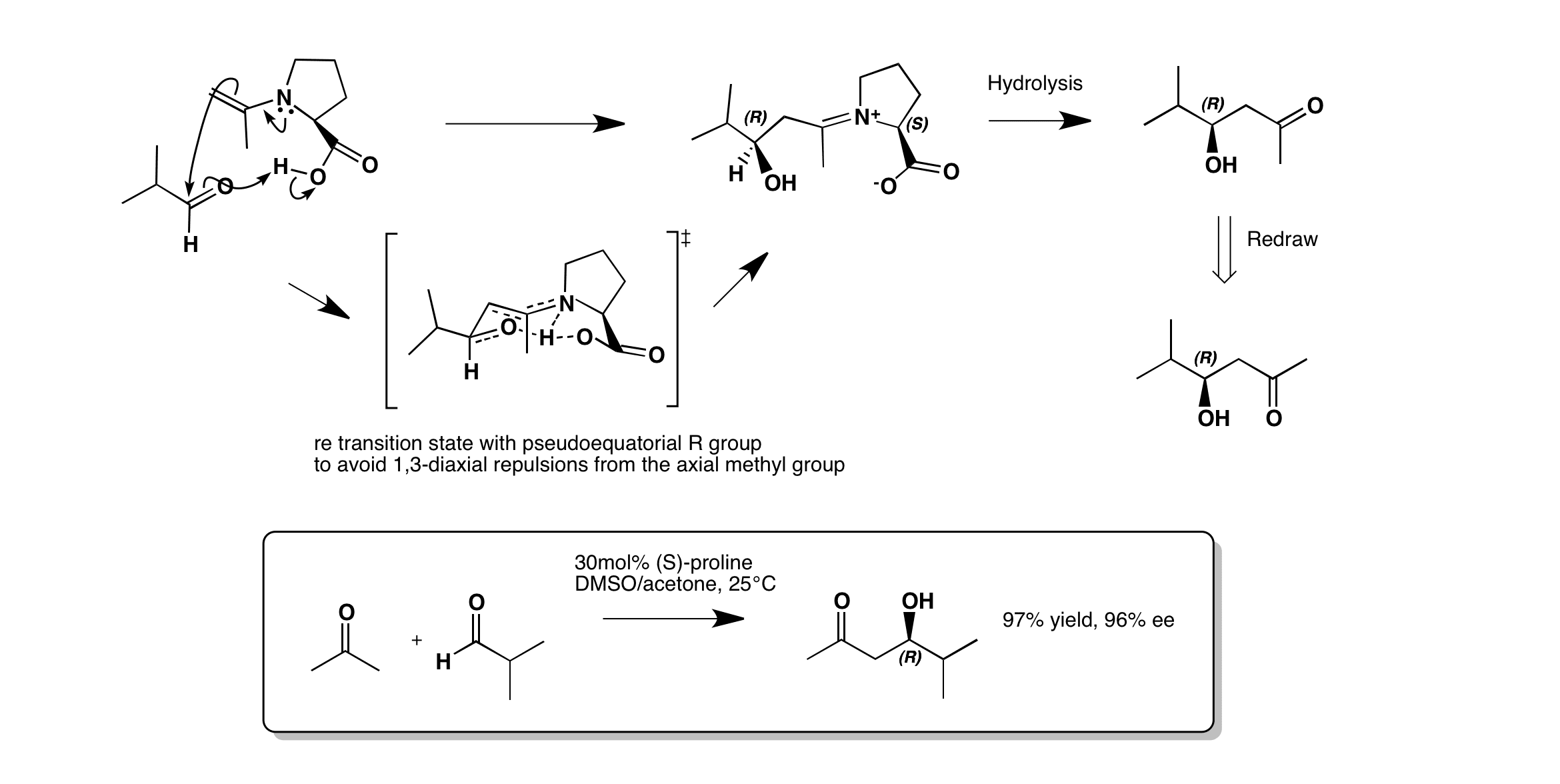
The reaction goes via an enamine, which attacks the aldehyde Re-face to form the R-product. In the transition state the isopropyl group is pseudoequatorial to minimise the 1,3-diaxial repulsions from the axial methyl group alpha to the nitrogen. This forms the R-aldol product.
Click for more information on Re and Si.
B. List, R. A. Lerner and C. F. Barbas, J. Am. Chem. Soc., 2000, 122, 2395–2396.