Click on the orbitals in the diagram to show the HOMO or LUMO of the molecule
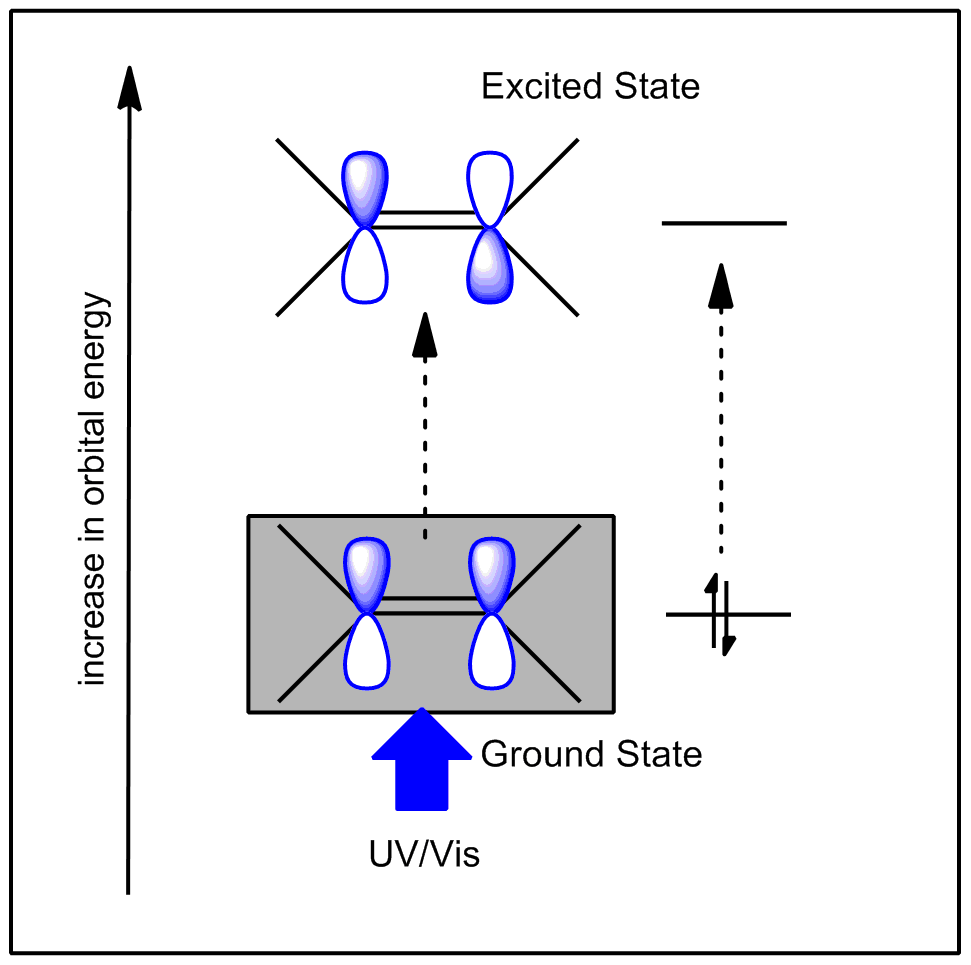 |
The energy needed to change the occupation of orbitals in a molecule (i.e. the energy required for electrons to make the transition from one orbital to another) is of the order of several electron volts (>100 kJ mol-1). This energy corresponds to the ultraviolet and visible regions of the electromagnetic spectrum. The more tightly bound the electrons are, the more energy is required to excite them. The highest energy transitions can require photons with energies in the X-ray region (>1×106 cm-1).
In the example below, we show the HOMO (Highest Occupied Molecular Orbital) and LUMO (Lowest Unoccupied Molecular Orbital) of the simple molecule ethylene (ethene). You can rotate the molecule by clicking and holding the window, and moving the mouse.
A molecular orbital description of UV spectra of polyenes is available.