Click the structures and reaction arrows in sequence to view the 3D models and animations respectively
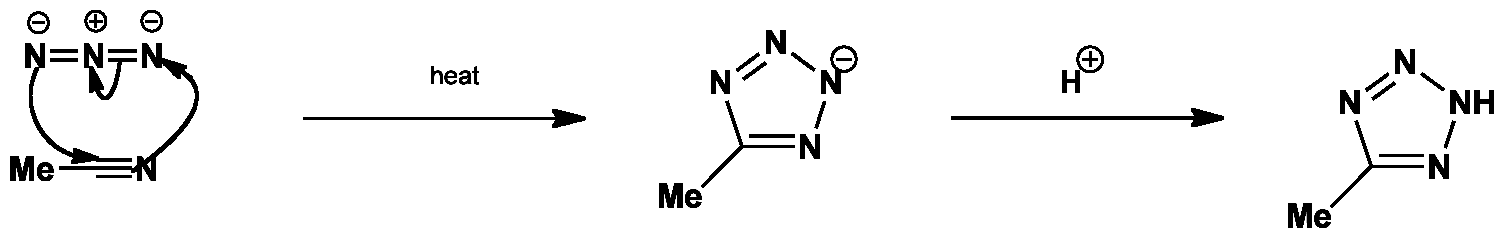
The azide and the nitrile undergo 1,3-dipolar cycloaddition to give the tetrazole anion. The anion is then protonated on workup to give the final tetrazole product.
S. Das, S. Santra, P. Mondal, A. Majee and A. Hajra, Synthesis (Stuttg)., 2016, 48, 1269–1285.