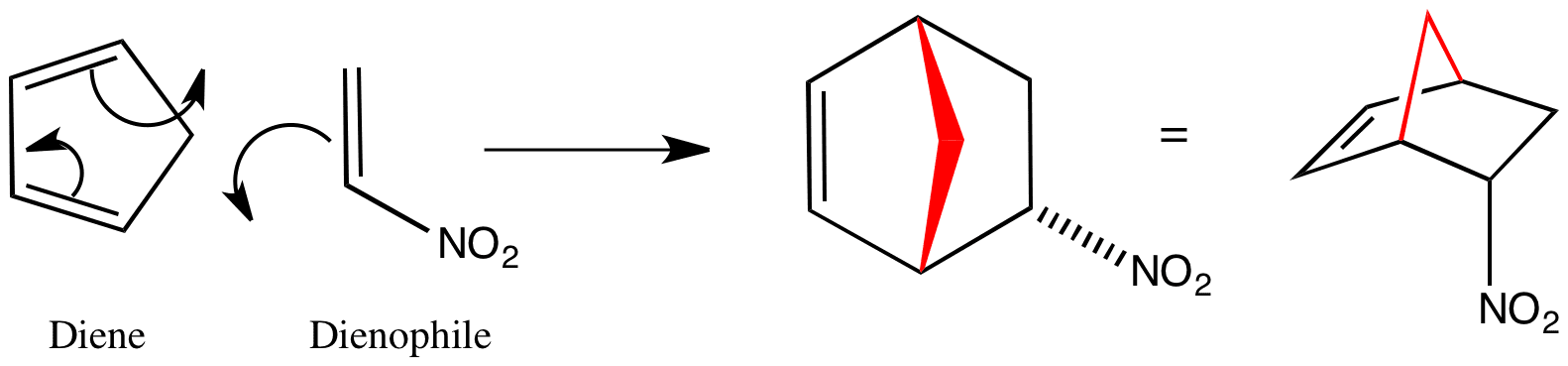
Click the structures and reaction arrows in sequence to view the 3D models and animations respectively
Diels-Alder reactions are cycloadditions which occur between a diene and a conjugated alkene, known as the dienophile.
Two sigma bonds are formed or broken in one concerted step – there are no intermediates at all. This type of reaction is known as a pericyclic reaction. As seen above, the endo transition state leads to the nitro group being opposite the CH2 bridge – for more information about endo and exo see this page.
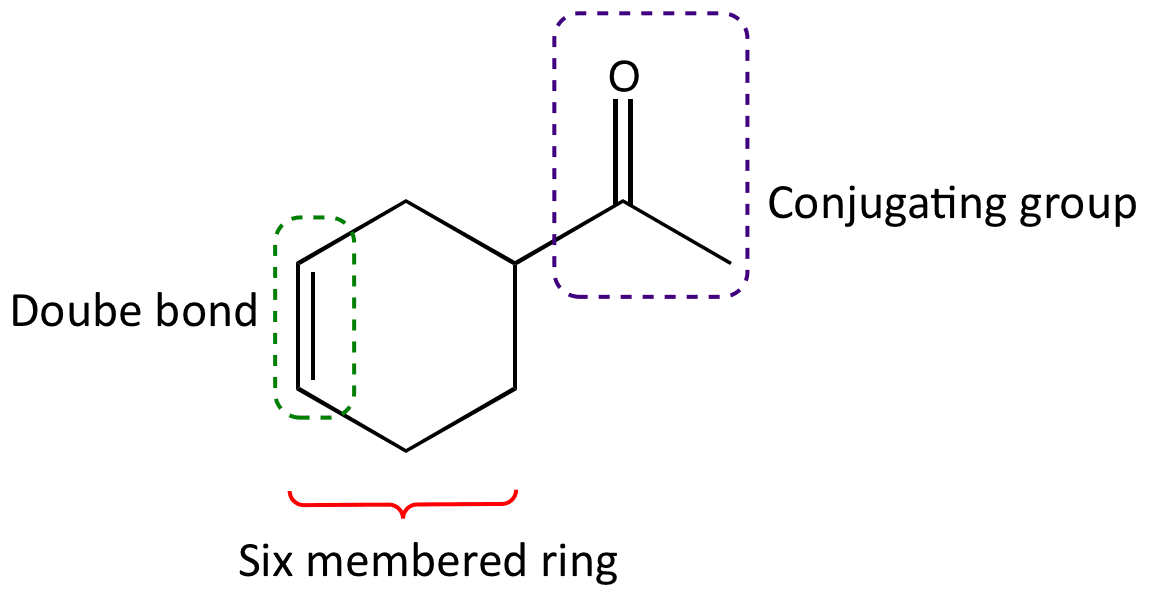
Recognising a Diels-Alder product is easy. Look for a six-membered ring with a double bond inside the ring and a conjugating group outside the ring on the opposite side to the double bond.