Click the structures and reaction arrows to view the 3D models and animations respectively
NOTE: Important charges and non-bonding electrons are shown throughout the animation except during the transition phase
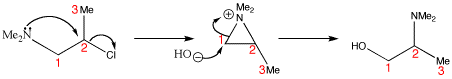
In this rearrangement OH replaces Cl but with a change in the molecular structure. The substitution goes with complete rearrangement – the amine ends up attatched to a different carbon. The reaction starts off looking like a neighbouring group participation. The intermediate is an aziridinium ion (nitrogen analogue of an epoxide). The hydroxide ion chooses to attack the less hindered terminal carbon 1, and a rearrangement results – the amine has migrated from carbon 1 to carbon 2.
W. R. Brode and M. W. Hill, J. Am. Chem. Soc., 1947, 69, 724–724.