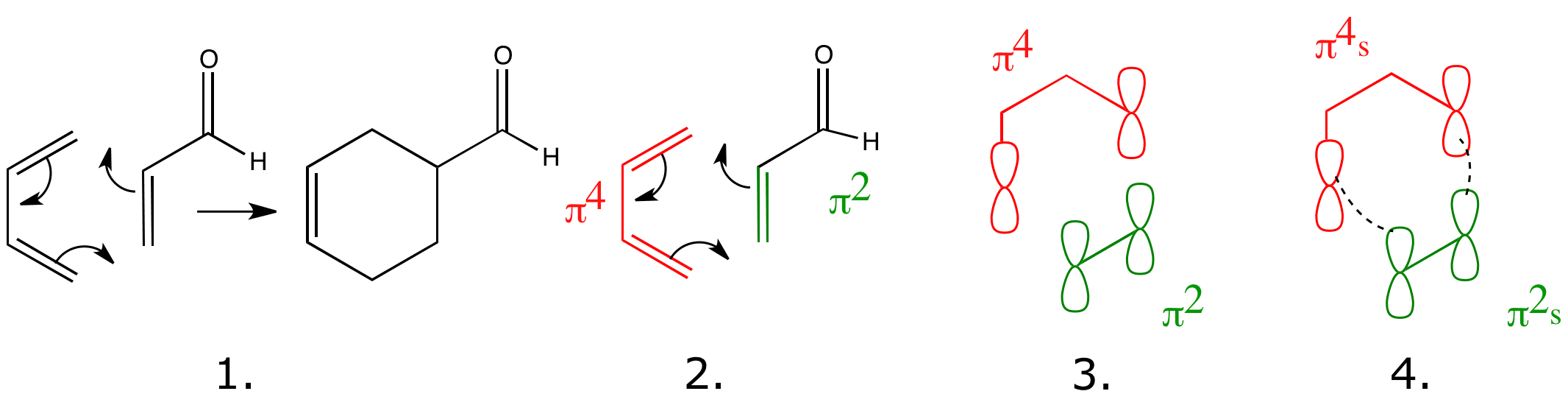
The best way to make sense of the Woodward-Hoffmann rules is to try using them.
Click on the numbers and structures below to see how this works for the Diels-Alder reaction.

‘In allowed thermal pericyclic reactions the number of (4q+2)s and (4r)a components must be odd.’
Let’s break it down:
- A component is a bond or orbital that takes part in the reaction as one unit. Both the diene and the dienophile are each one single component. We label the components by the type and number of electrons there are in the component.
- The suffix ‘s‘ stands for a suprafacial component – forms new bonds on the same face at both ends.
- The suffix ‘a‘ stands for a antarafacial component – forms new bonds on opposite faces at each end.
- Only suprafacial components with (4q+2) electrons is counted by the Woodward-Hoffman rule. The q is simply an integer 1,2,3 . . etc. So only suprafacial components with 2, 6 or 10 electrons count.
- Only antarafacial components with (4r) electrons is counted by the Woodward-Hoffman rule. Again r is simply an integer 1,2,3 . . etc. So only antarafacial components with 0, 4 or 8 electrons count.
For allowed pericyclic reactions:
| (Number of suprafacial components with 2, 6 or 10 electrons) | |
| + | |
| (Number or antarafacial components with 0, 4 or 8 electrons) | |
| =ODD NUMBER | |
Number of (4q+2)s components = 1, Number of (4r)a components = 0
No. of (4q+2)s + No. of (4r)a components = 0 +1 =1 = AN ODD NUMBER
The Diels-Alder reaction is an allowed thermal pericyclic reaction.
R. B. Woodward and R. Hoffmann, Angew. Chemie Int. Ed. English, 1969, 8, 781–853.