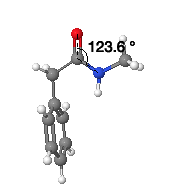
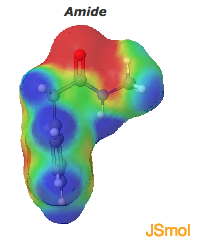
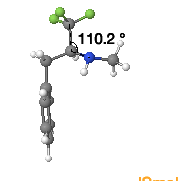
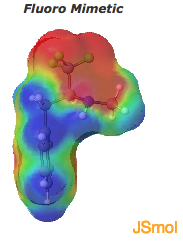
Click the images to compare them in 3D
Mimicry between trifluoroethyl-amine derivative and amides comes about due to their geometrical and electronic similarity. The C-C-N bond angle of the fluorinated amide mimetic is very similar to the amide, making them geometrically similar.
The CF3 fragment in the mimetic is also highly electron withdrawing, which reduces the basicity of the amine such that its hydrogen bond donating property is more like that of an amide. This can be seen in the electron density maps of these examples. Fluorination can lead to polarization of a C-H bond which allows the carbons hydrogen to function as a hydrogen bond donor.