Click the structures and reaction arrows in sequence to view the 3D models and animations respectively
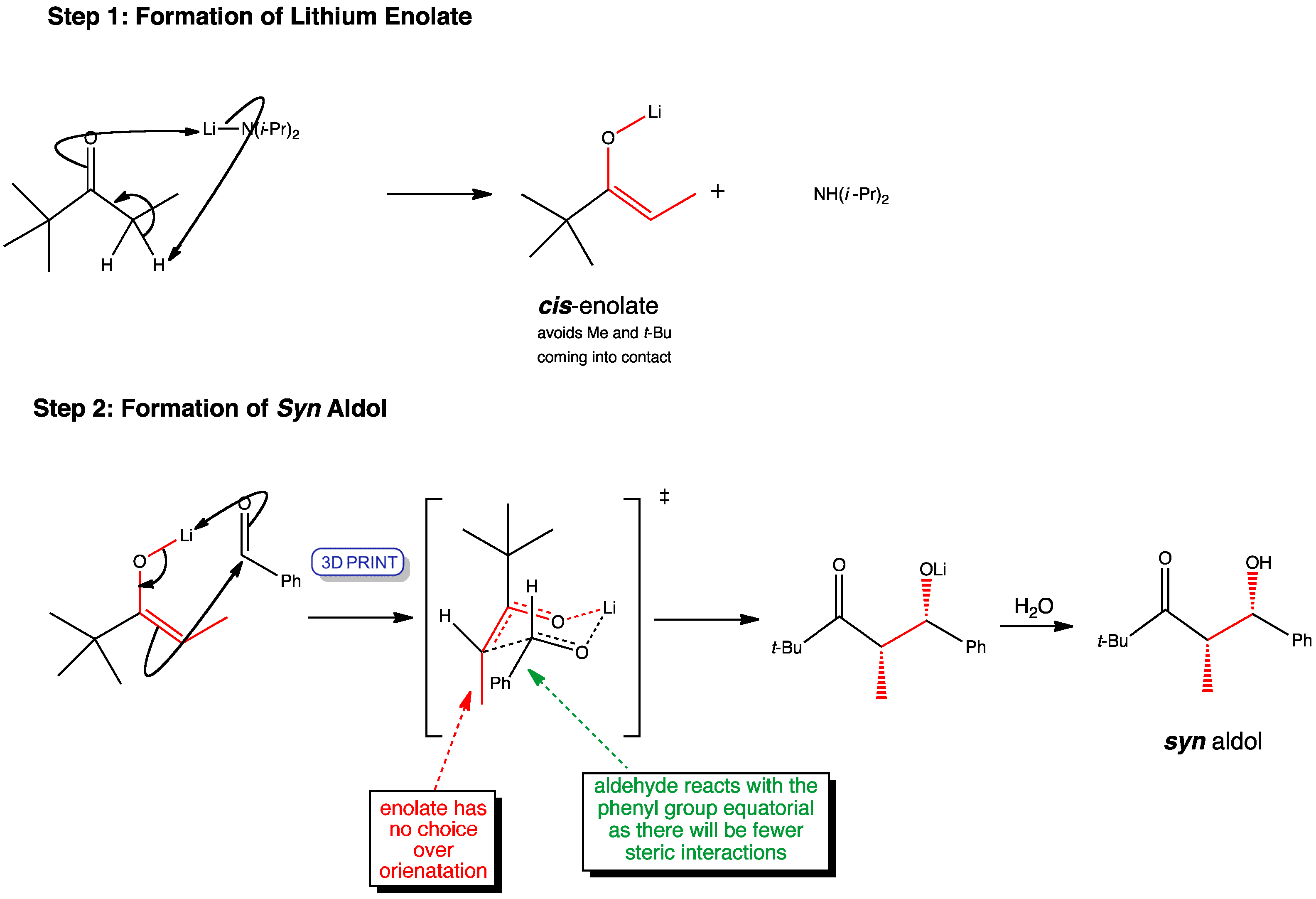
The bulky t-butyl group attached to the carbonyl controls the enolate geometry. Only a single geometrical isomer is possible as the cis conformation reduces the steric interactions between the methyl and t-butyl groups. The cis enolate then reacts with the aldehyde to give only the syn aldol product via a chair-like transition state and the aldehyde phenyl equatorial.