NOTE: Important charges and non-bonding electrons are shown throughout the animation except during the transition phase
Click the structures and reaction arrows to view the 3D models and animations respectively
Cis-trans isomerisation and solvent reorganisation throughout the reaction are not shown explicitly.
For simplicity animations are shown with PH3 and SnH3 instead of PR3 and SnR3 respectively.
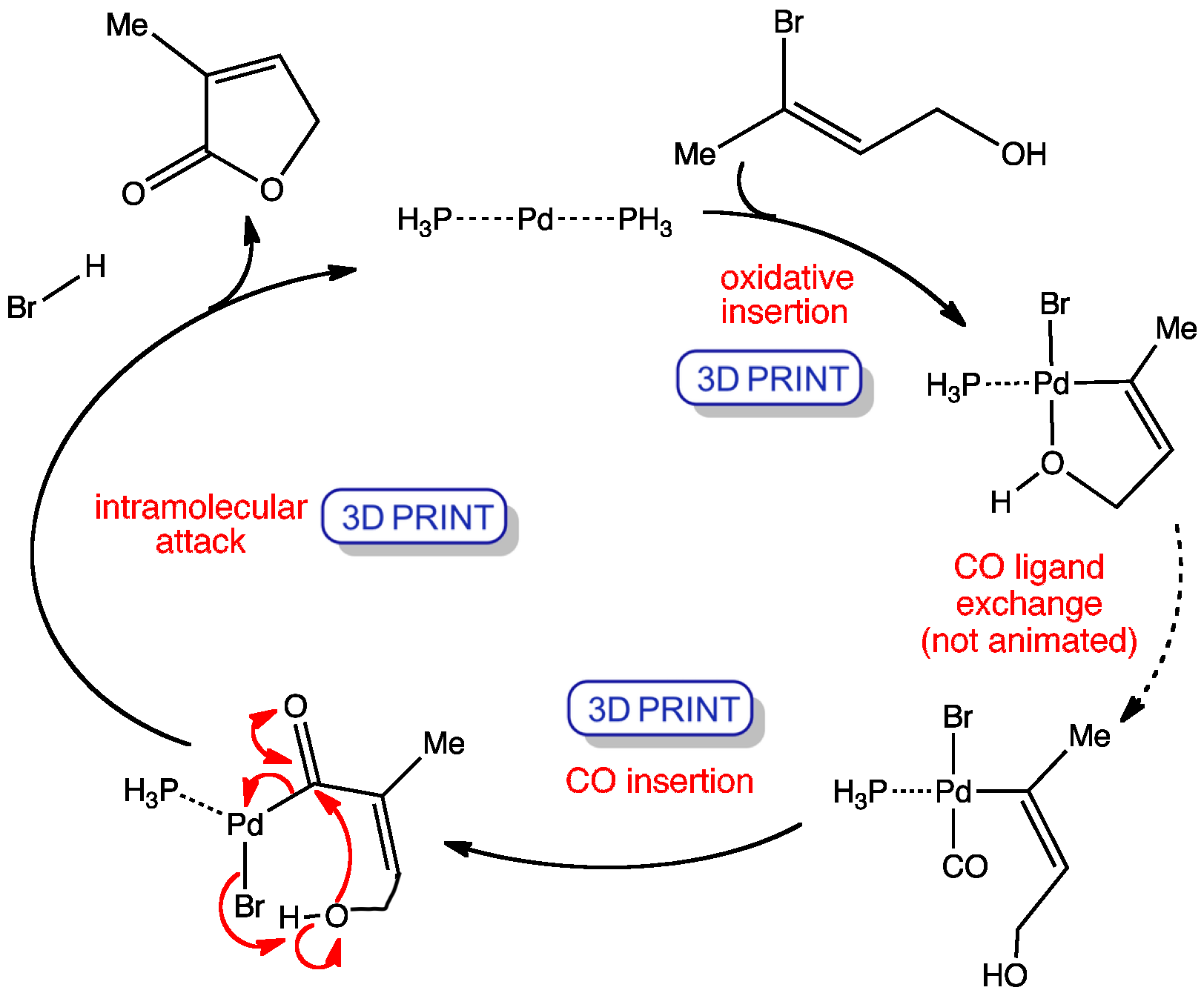
When there is a nucleophilic group present in the starting material such as an amine or hydroxy group, then this nucleophile can attack the acyl palladium intermediate to ultimately form a butenolide.
The mechanism involves the oxidative addition of the vinyl bromide to give a palladium(II) intermediate, which undergoes a a carbonylation reaction. The carbon monoxide first exchanges for one of the phosphines and then rapid insertion produces an acyl palladium (II) complex. The hydroxy nucleophile then attacks the carbonyl coordinated to the palladium releasing the lactone product and regenerating the palladium(0) catalyst.
G. T. Crisp and A. G. Meyer, J. Org. Chem., 1992, 57, 6972–6975.