Click the structures and reaction arrows in sequence to view the 3D models and animations respectively
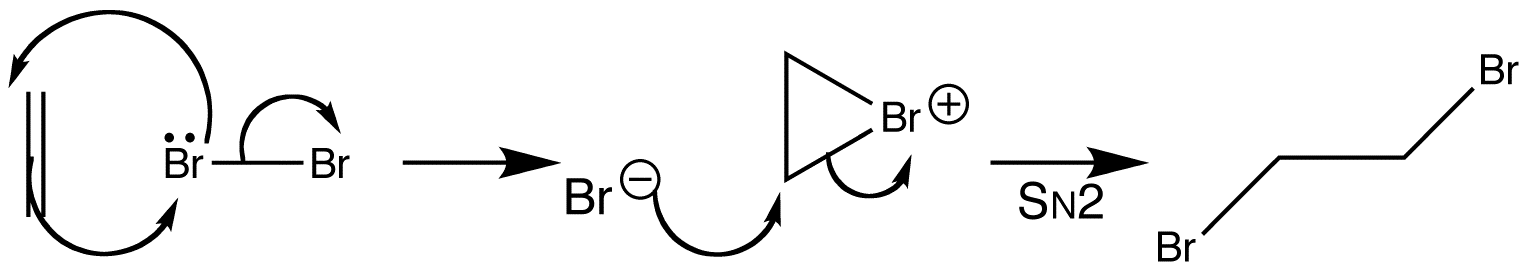
A classic test for alkenes is that they turn a brown aqueous solution of bromine colourless. Alkenes react with bromine to decolourize bromine water. Alkenes react in non-polar solvents to give a dibromoalkane product. Simple, unconjugated alkenes are nucleophilic and react with electrophiles.
In this case, the alkene is the nucleophile, and its HOMO is the C=C π bond. When it reacts with bromine, the alkenes filled π orbital (the HOMO) will interact with the bromines empty σ* orbital to give a product. The highest electron density in the π orbital is right in the middle, between the two carbon atoms, so this is where we expect the bromine to attack. The only way the π HOMO can interact in a bonding manner with the σ* LUMO is if the bromine approaches end-on, and this is how the product forms. The symmetrical three-membered ring intermediate is called a bromonium ion.
I. Roberts and G. E. Kimball, J. Am. Chem. Soc., 1937, 59, 947–948.