NOTE: Important charges and non-bonding electrons are shown throughout the animation except during the transition phase
Click the structures, reaction arrows, and orbital images to view the 3D models, animations, and animated molecular orbitals respectively
Cis-trans isomerisation and solvent reorganisation throughout the reaction are not shown explicitly.
For simplicity animations are shown with PH3 instead of PR3.
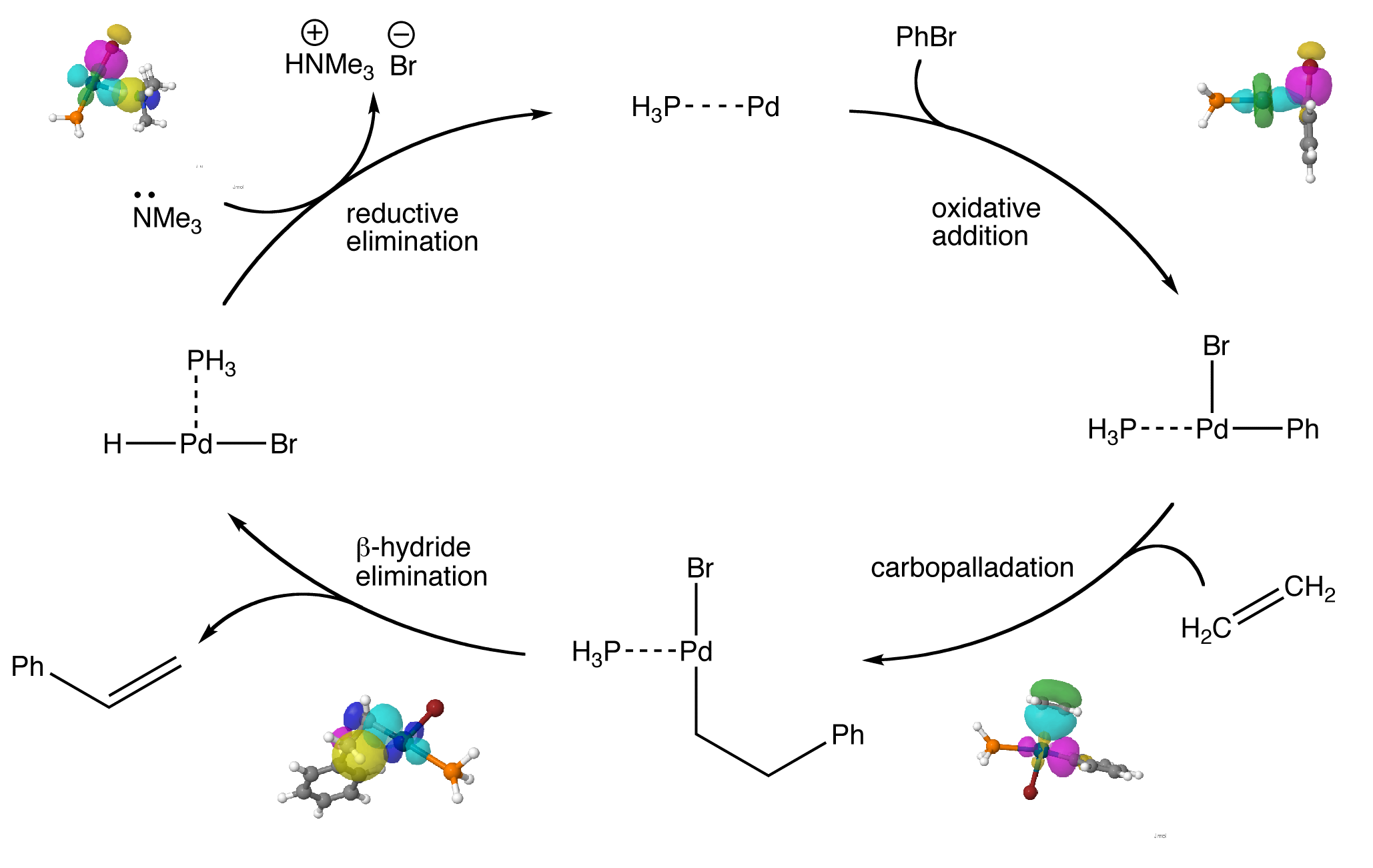
The Mizoroki-Heck reaction, commonly known as the Heck reaction, is the palladium-catalysed addition of aryl, vinyl, or substituted vinyl groups to organic halides or triflates.
The mechanism involves the oxidative addition of the halide, migratory insertion (or carbopalladation) of the olefin, and β-hydride elimination to form the product. The palladium(0) catalyst is then regenerated using a base in the reductive elimination step.
Animations based on P. Surawatanawong, Y. Fan, M.B. Hall, J. Organomet. Chem. 693 (2008) 1552-1563.
I. P. Beletskaya and A. V. Cheprakov, Chem. Rev., 2000, 100, 3009–3066.