NOTE: Important charges and non-bonding electrons are shown throughout the animation except during the transition phase
Click the structures and reaction arrows to view the 3D models and animations respectively
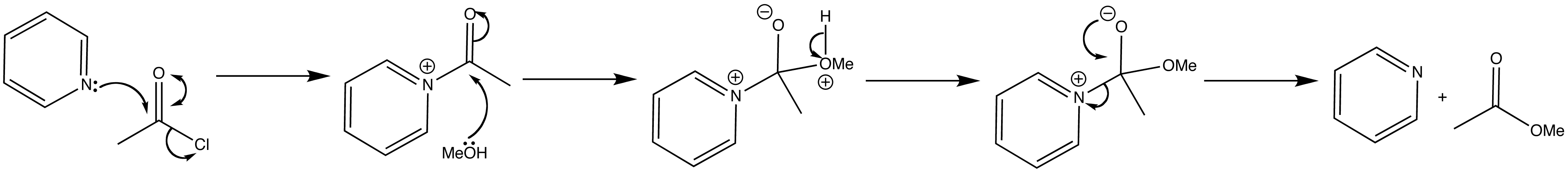
Pyridine is a reasonable nucleophile for carbonyl groups and is often used as a catalyst in acylation reactions. The nitrogen atom in pyridine is nucleophilic because the lone pair of electrons on nitrogen cannot be delocalised around the ring.
Esters are often made in pyridine solution from alcohols and acid chlorides.