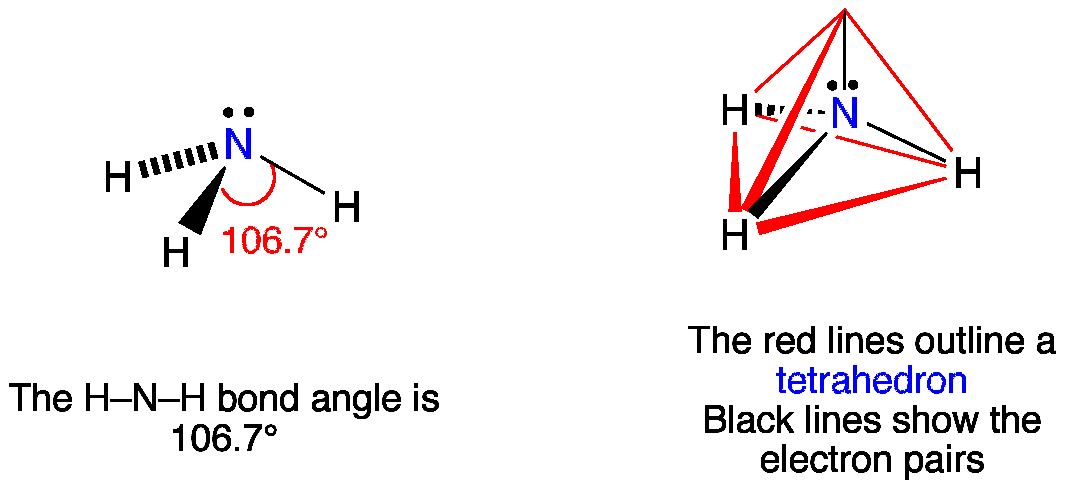
Ammonia has 4 regions of electron density around the central nitrogen atom (3 bonds and one lone pair). These are arranged in a tetrahedral shape. The resulting molecular shape is trigonal pyramidal with H-N-H angles of 106.7°.
Related structures H2O | NH3 | CH4 | PF5 |SF4 |ClF3 | SF6 | XeF4