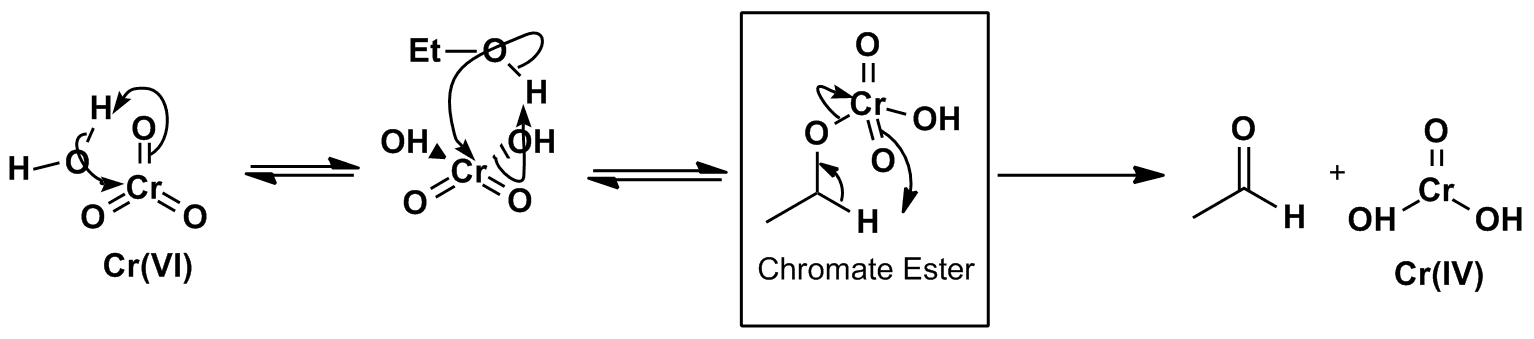
Click the structures and reaction arrows in sequence to view the 3D models and animations respectively
The Cr2O72- ion (Cr VI) forms HCrO4– in acidic aqueous solution, which can eliminate a molecule of water to give the reagent CrO3. This then oxidises alcohols to a aldehydes and ketones via the chromate ester intermediate. The reduced chromium Cr (IV) can undergo series of reactions bringing it ultimately to the Cr (III) oxidation state.
K. Bowden, I. M. Heilbron, E. R. H. Jones and B. C. L. Weedon, J. Chem. Soc., 1946, 0, 39.
E. J. Corey and G. Schmidt, Tetrahedron Lett., 1979, 20, 399–402.