Click the structures and reaction arrows in sequence to view the 3D models and animations respectively
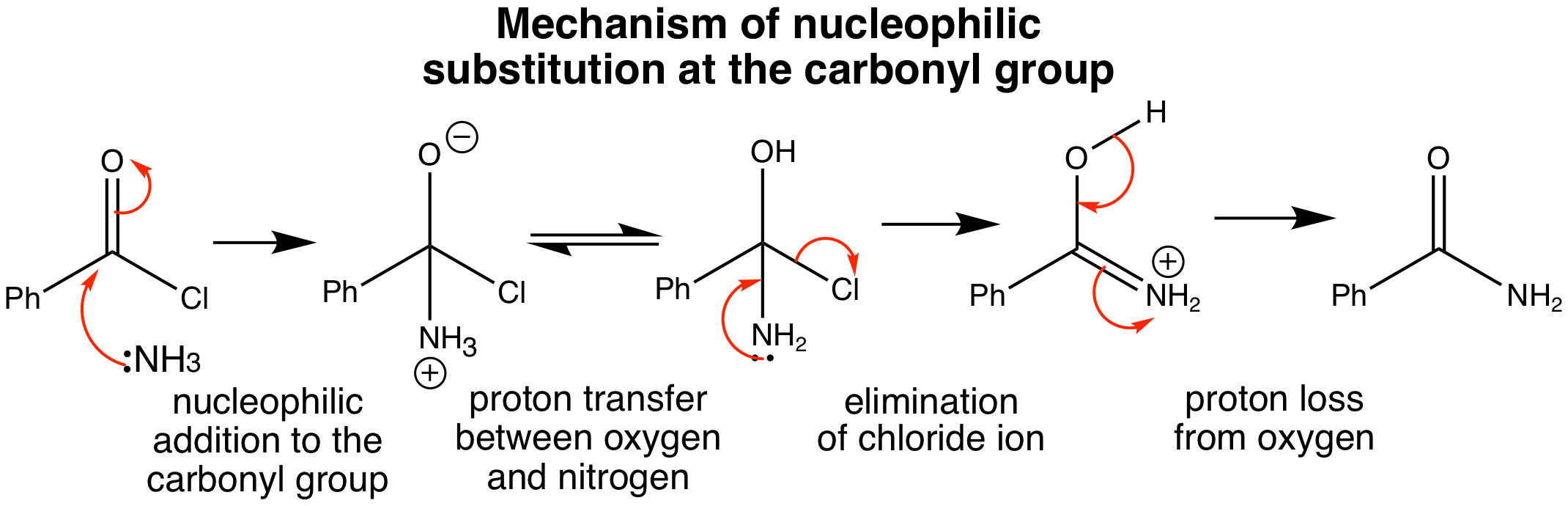
Above is an example of a nucleophilic substitution reaction at a carbonyl group, in which the phenyl and carbonyl groups remain in the molecule, but the chloride group is replaced with the amine group. The molecule of ammonia acts as the nucleophile with the chloride atom as the leaving group.
C. A. G. N. Montalbetti and V. Falque, Tetrahedron, 2005, 61, 10827–10852.