NOTE: Important charges and non-bonding electrons are shown throughout the animation except during the transition phase
Click the structures and reaction arrows in sequence to view the 3D models and animations respectively
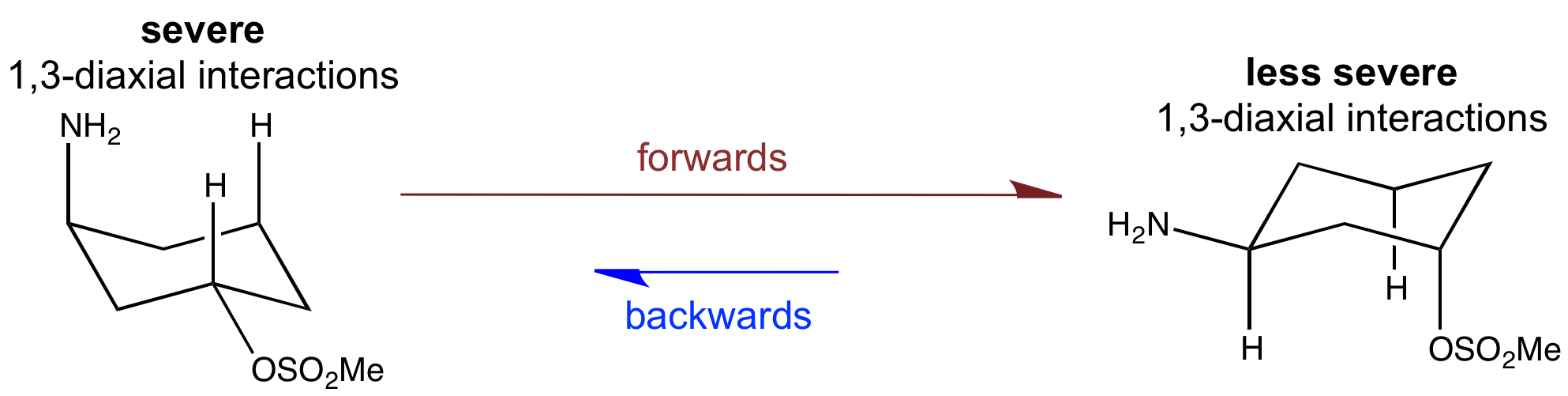
In the cis isomer both substituents can be equatorial. However, in the trans isomer, one substituent has to be axial. This will mainly be the mesylate group, since the NH2 group suffers greater 1,3-diaxial interactions.
Click the links below to view animations for both trans isomers:
Back to stereochemistry main page
U. Burckhardt, C. A. Grob and H. R. Kiefer, Helv. Chim. Acta, 1967, 50, 231–244.