– 8:8 (cubic)
Non close-packed, arrangement of Cl with Cs in cubic holes.
Polyhedra – Face-sharing and
cubes
All of the cubic holes in a simple cubic packing are occupied.
Both ions show cubic coordination (CN = 8)
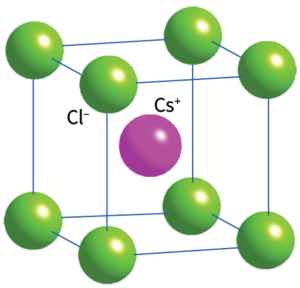
The CsCl structure adopted by CsCl, CsBr, and CsI under normal conditions.
Related Structure: Body-centred cubic (bcc)