Click the structures and reaction arrows in sequence to view the 3D models and animations respectively
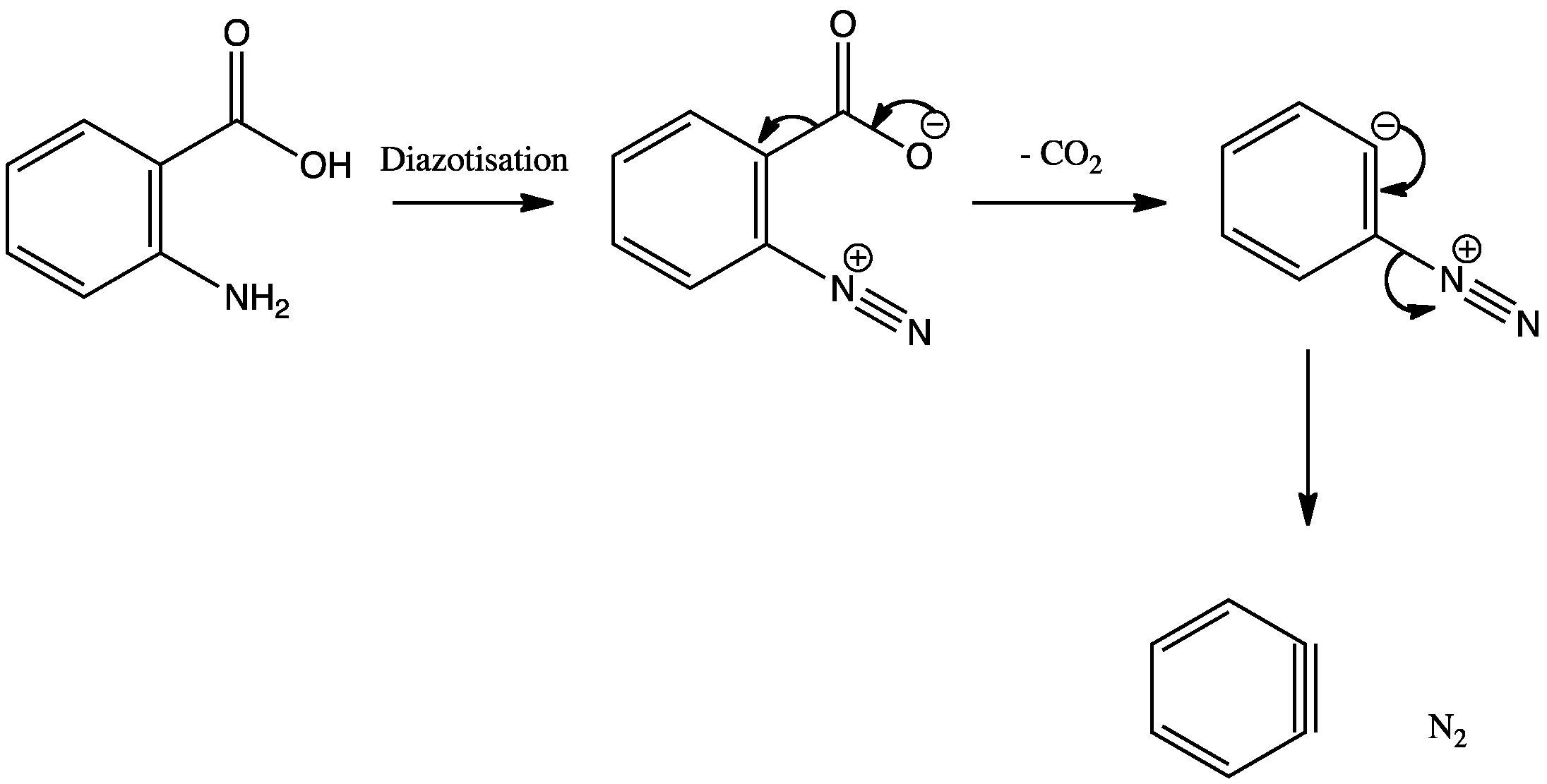
Diazotization of anthranilic acid produces a diazonium salt which can lose nitrogen and carbon dioxide to produce benzyne. Evidence for this rather unlikely looking intermediate is provided by the fact that it can be trapped in a Diels-Alder reaction. The benzyne intermediate also appears in the aromatic substitution reactions.
P. M. Tadross and B. M. Stoltz, Chem. Rev., 2012, 112, 3550–3577.