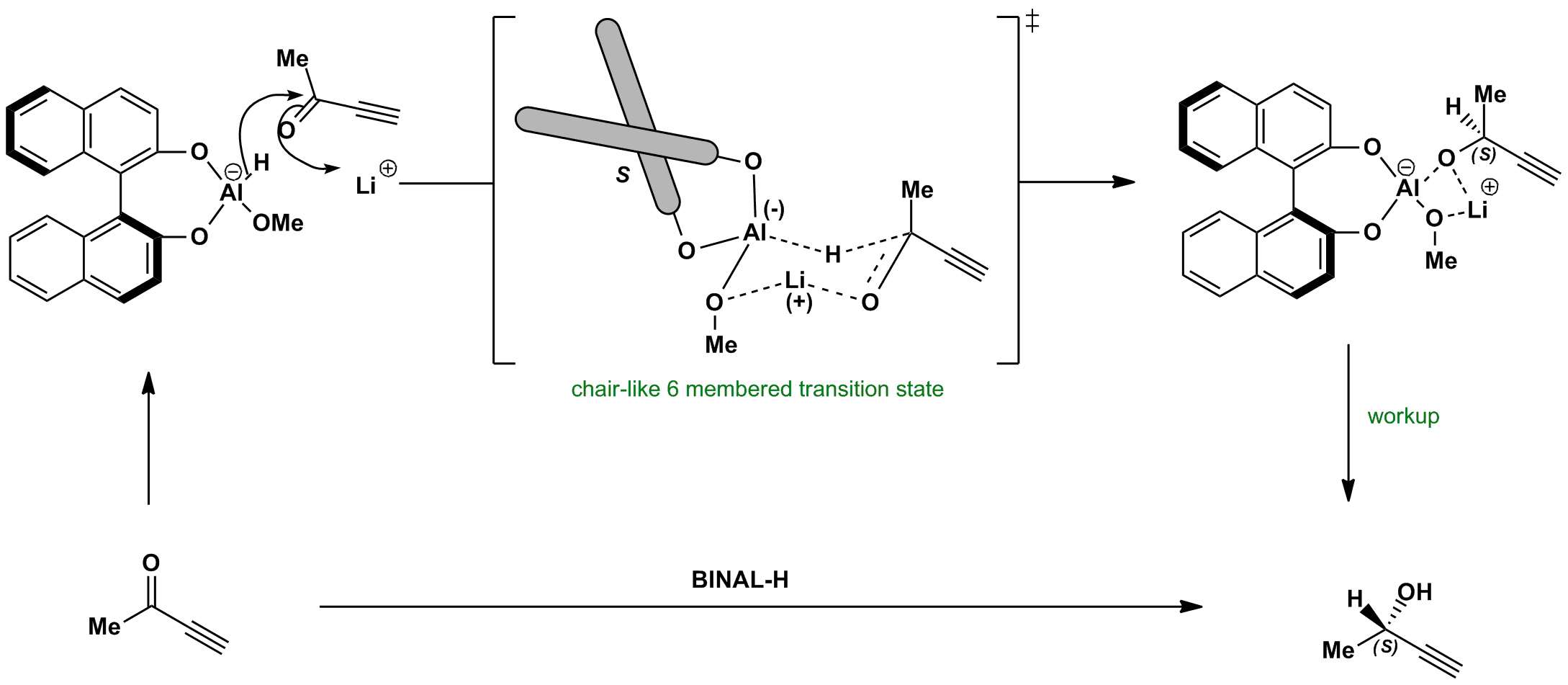
Click the structures and reaction arrows in sequence to view the 3D models and animations respectively
The unsaturated group (alkyne in this case) has an unfavourable orbital interaction with lone pairs on the BINOL oxygen, so it is equatorial. This gives only one face for the hydride to attack. Note the transition state is a distorted chair – this is due to the number of heteroatoms (atoms other than carbon) in the ring.
R. Noyori, I. Tomino, Y. Tanimoto and M. Nishizawa, J. Am. Chem. Soc., 1984, 106, 6709–6716.