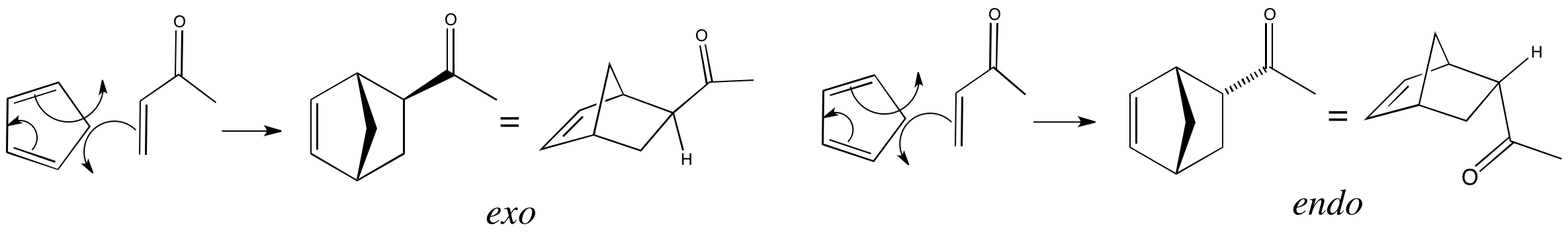
Click the structures and reaction arrows in sequence to view the 3D models and animations respectively
In this reaction only two possible diastereoisomers can be formed – exo and endo. We can work out which is the endo product using the method described, and this turns out to be the product with the carbonyl group on the opposite side to the CH2 bridge.