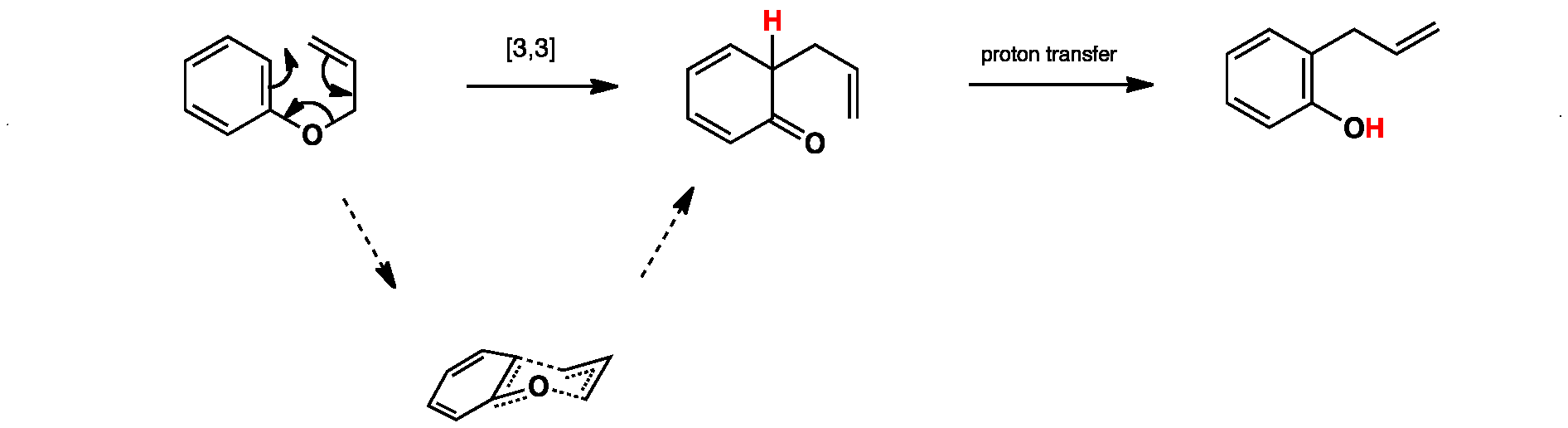
Click the structures and reaction arrows in sequence to view the 3D models and animations respectively
The first step in the reaction is a pericyclic [3,3]-sigmatropic rearrangement, which happens through a chair-like transition state. The second step is a simple proton transfer to regenerate aromaticity.
K. C. Majumdar and R. K. Nandi, Tetrahedron, 2013, 69, 6921–6957.