Click the structures and reaction arrows in sequence to view the 3D models and animations respectively
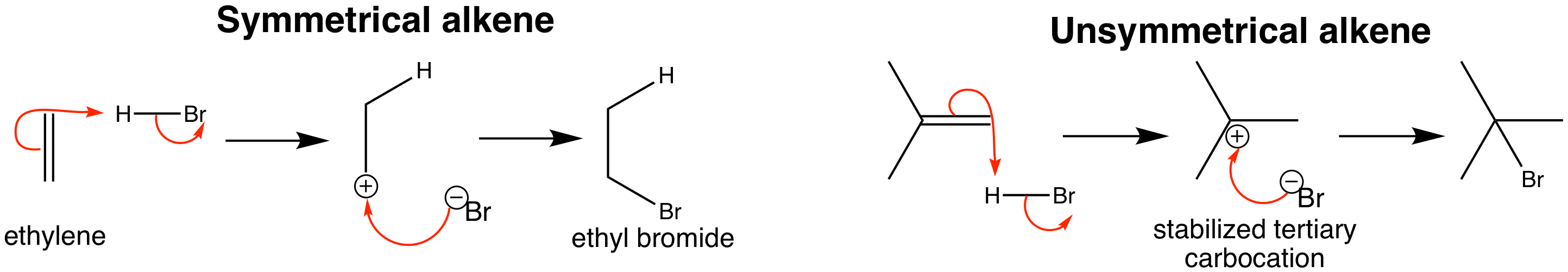
In some electrophilic addition reactions, such as those with HBr and an alkene, there is a choice as to which carbon ends up bonded to the H and which the Br. It is important to be able to predict and explain the reactions of unsymmetrical alkenes based on their structures. When HBr reacts with a nucleophile, it is attacked at the hydrogen and loses a bromide ion. Hydrogen cannot form a three-membered cation, so the reaction produces a carbocation. This carbocation then rapidly reacts with the bromide ion, so that overall, the HBr has been added across the alkene.
The bromine atom ends up on the more substituted carbon, which can be understood when you look at the mechanism. Given that a carbocation is formed, the positive charge prefers to reside on the more substituted carbon, giving a more stable tertiary cation as opposed to the alternative much less stable primary cation, which is not formed.