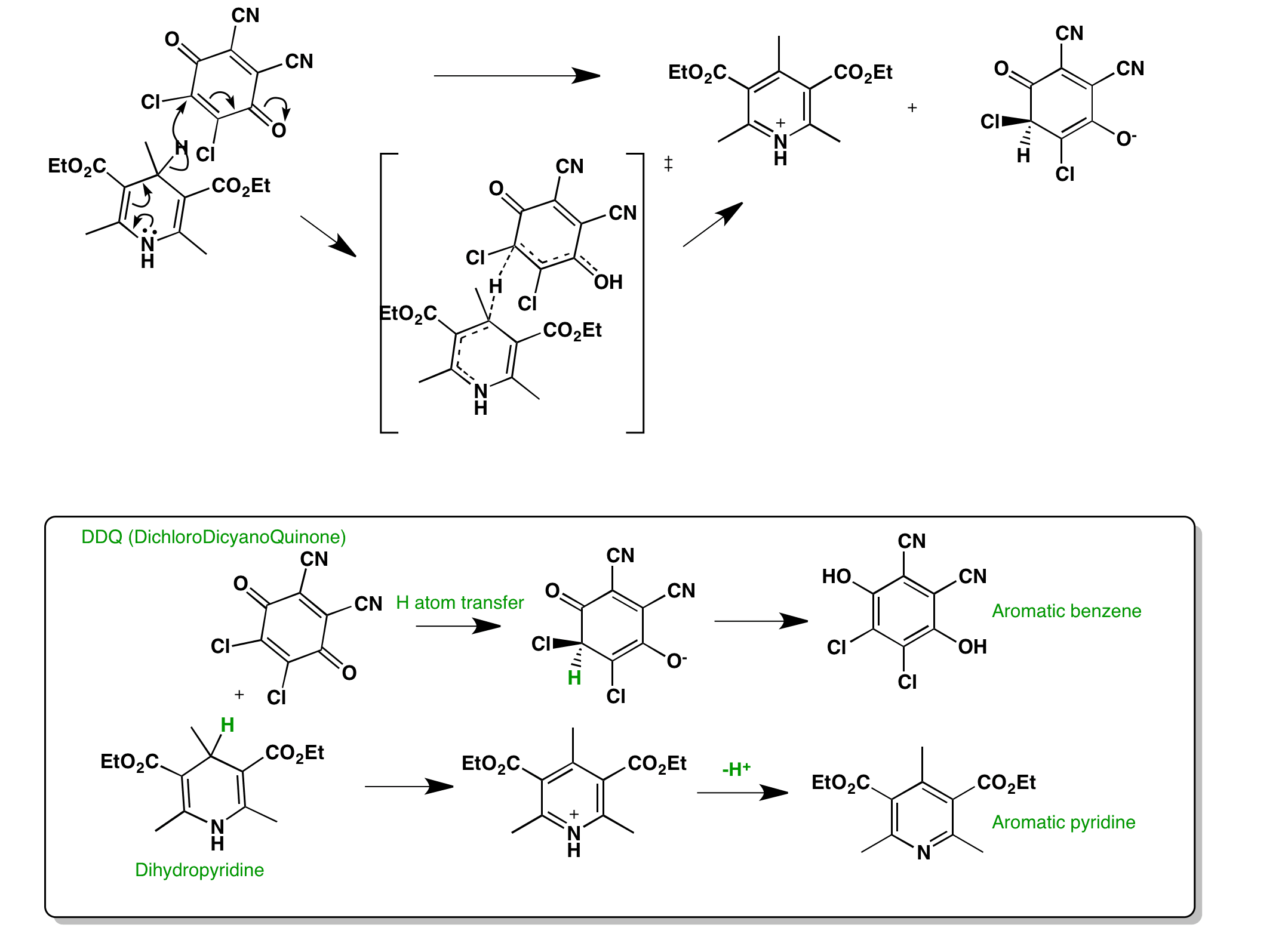
Click the structures and reaction arrows in sequence to view the 3D models and animations respectively
Dihydropyridines are oxidised by various oxidants such as quinones e.g. 2,3-Dichloro-5,6-dicyano-1,4-benzoquinone (DDQ) by transferring a hydride from the pyridine to DDQ. Subsequent proton exchange leads to the formation of two aromatic products (quinol and pyridine ring). This is the last stage of the Hantzsch pyridine synthesis.
X.-Q. Zhu, B.-J. Zhao and J.-P. Cheng, J. Org. Chem., 2000, 65, 8158–8163.