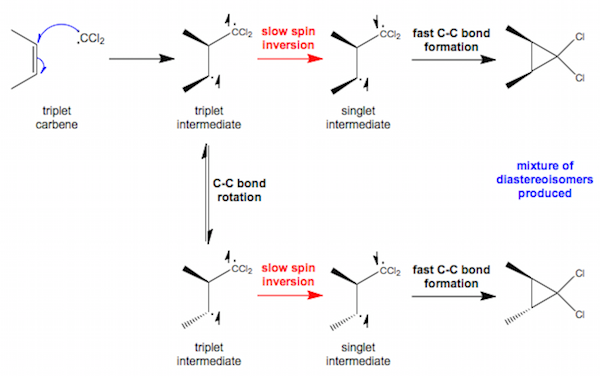
NOTE: Electron spins are labelled on the relevant atoms using up and down facing arrows, and the movement of radicals is represented using blue curly arrows
Click the structures and reaction arrows in sequence to view the 3D models and animations respectively
For triplet carbenes the reaction is nonstereospecific, so the mechanism of the reaction must therefore be different. In fact, a concerted reaction is impossible for triplet carbenes because of the spins of the electrons involved. After the carbene adds to the alkene in a radical reaction, the diradical (triplet) intermediate must wait until one of the spins inverts so that the second C-C bond can be formed with paired electrons. This intermediate lives long enough for C-C bond rotation and loss of stereochemistry.
Back to alkene insertion main page
P. de Frémont, N. Marion and S. P. Nolan, Coord. Chem. Rev., 2009, 253, 862–892.