Click the structures and reaction arrows in sequence to view the 3D models and animations respectively
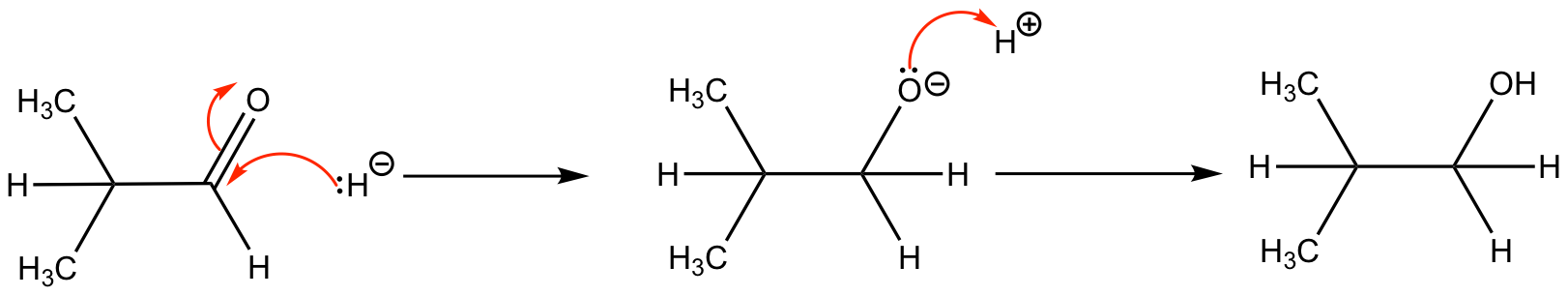
As has been previously discussed, the C=O bond is polar bond as there is a difference in electronegativities (oxygen is more electronegative than carbon), hence oxygen has a greater share of the bonding electrons. These polar bonds are easily reduced by a hydride (H–) ion. Under normal circumstances, NaBH4 can be used, but certain reactions require anhydrous LiAlH4.
This reaction shows a hydride attacking the electron deficient carbon forming an oxyanion, this oxyanion is then attacked by a proton to form a stable product.
While this mechanism is sufficient at A Level, for those looking to further study in chemistry or are interested, a more detailed mechanism is available.