Click the structures and reaction arrows in sequence to view the 3D models and animations respectively
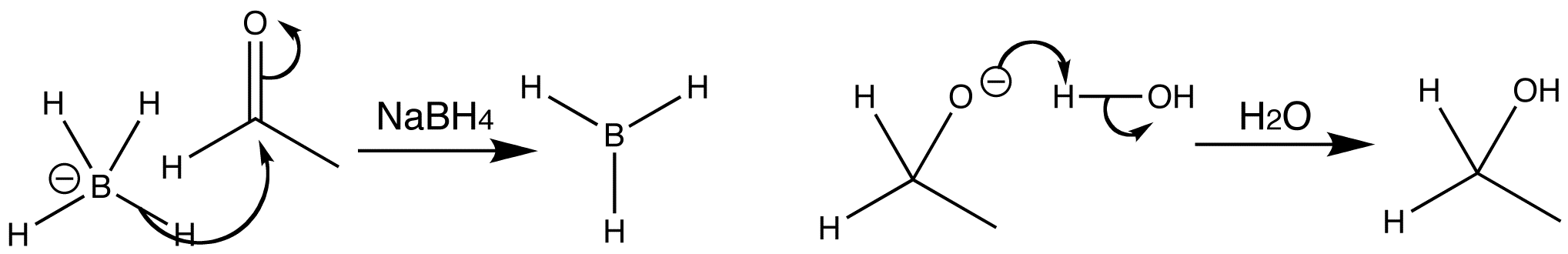
Hydride addition to a carbonyl group can be achieved with compounds containing nucleophilic hydrogen atoms, and is known as a reduction reaction. One such compound is sodium borohydride. The reaction involves the carbonyl group behaving as the electrophile, and a pair of electrons from one of the B-H bonds being transferred to the carbon atom of the C=O group. The reaction can be regarded as a ‘hydride transfer’.
D. C. Wigfield, Tetrahedron, 1979, 35, 449–462.