Click the structures and reaction arrows in sequence to view the 3D models and animations respectively
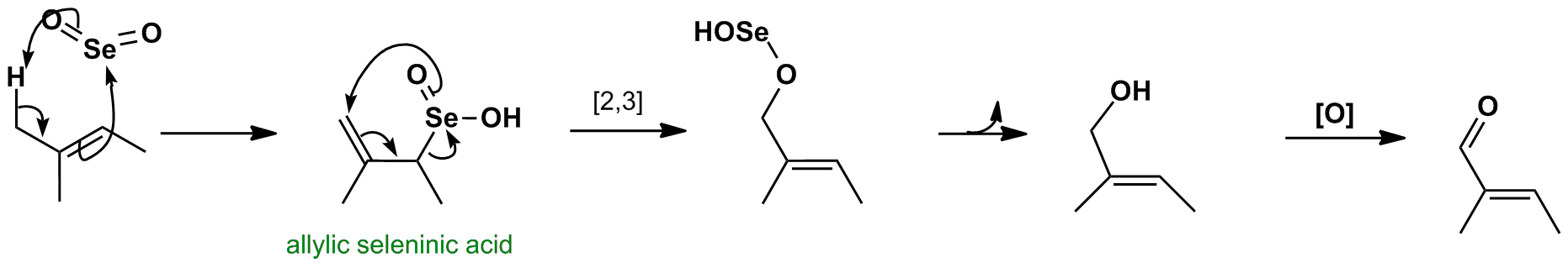
The first step is a cycloaddition similar to the carbonyl ene reaction. The allylic seleninic acid produced in the first step undergoes a [2,3]-sigmatropic rearrangement to reinstate the double bond position. Rapid decomposition of the selenium (II) intermediate leads to an allylic alcohol. Oxidation can continue to give the α,β-unsaturated carbonyl product (not animated).
A. Nakamura and M. Nakada, Synthesis (Stuttg)., 2013, 45, 1421–1451.