Click the structures and reaction arrows in sequence to view the 3D models and animations respectively
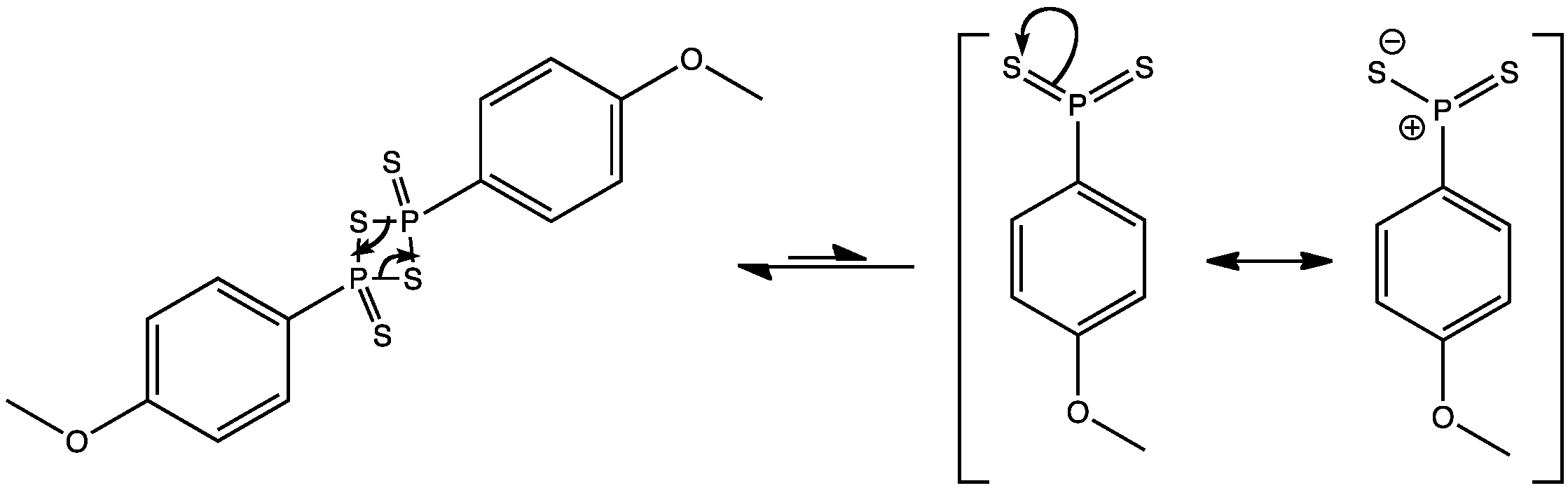
Lawesson’s reagent is a very convenient thionating agent, however, before it can react with carbonyl groups, it must break apart into two symmetrical molecules. In solution, Lawesson’s reagent is in equilibrium with the monomeric form which can be represented as either of the forms shown above.
Next step Thioketone formation
T. Ozturk, E. Ertas and O. Mert, Chem. Rev., 2007, 107, 5210–5278.