NOTE: Important charges and non-bonding electrons are shown throughout the animation except during the transition phase
Click the structures and reaction arrows to view the 3D models and animations respectively
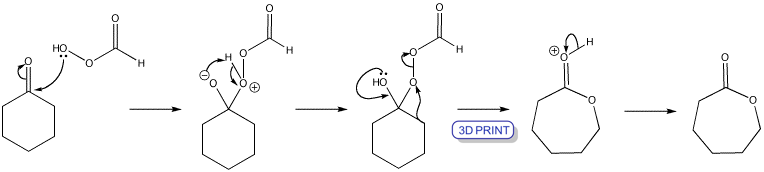
A. Baeyer and V. Villiger, found that treating a ketone with a peroxy-acid (RCO3H) can produce an ester. An oxygen atom is ‘inserted’ next to the carbonyl group. The oxygen-oxygen single bond is very weak and monovalent oxygen cannot bear to carry a positive charge. Once the peracid has added, loss of carboxylate is concerted with a rearrangement driven by formation of a carbonyl group.
G.-J. ten Brink, I. W. C. E. Arends and R. A. Sheldon, Chem. Rev., 2004, 104, 4105–4124.