NOTE: Important charges and non-bonding electrons are shown throughout the animation except during the transition phase
Click the structures and reaction arrows in sequence to view the 3D models and animations respectively
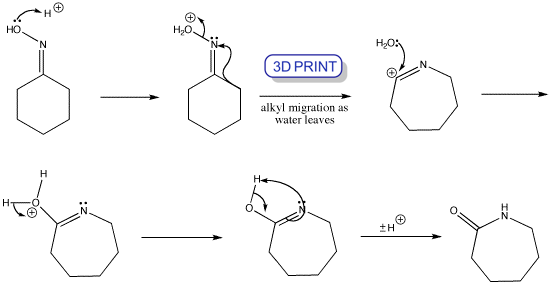
The mechanism of the Beckmann rearrangement follows the same pattern as a pinacol reaction – acid converts the oxime OH into a leaving group, and an alkyl group migrates on to the nitrogen as water departs. The product cation is then trapped by water to give an amide. This rearrangement is not confined to cyclic oximes, and other ways of converting OH to a leaving group also work, such as PCl5, SOCl2, and other acyl or sulfonyl chlorides.