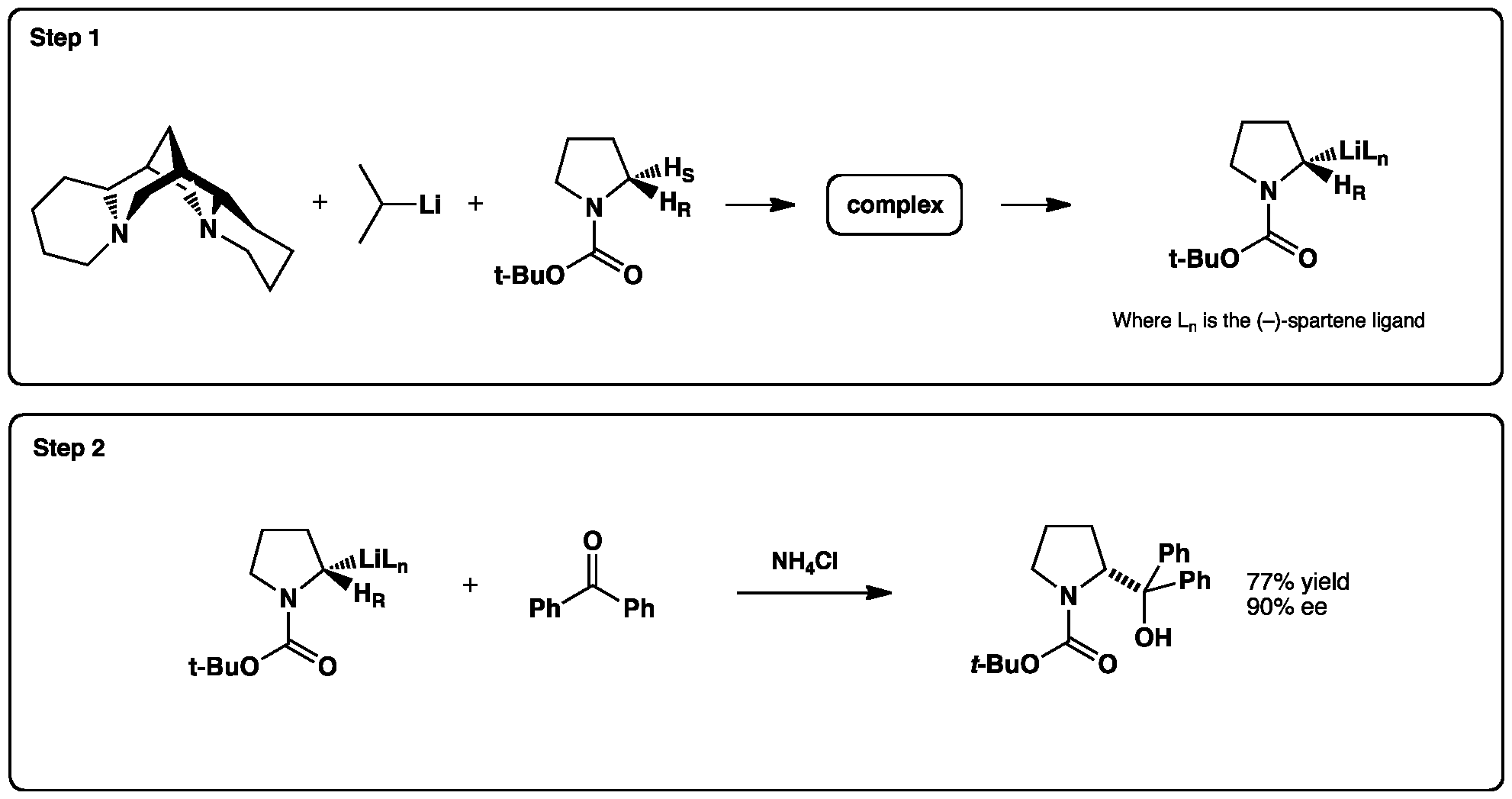
Deprotonation of Boc-pyrrolidine with an Alkyllithium in the Presence of a Chiral Diamine
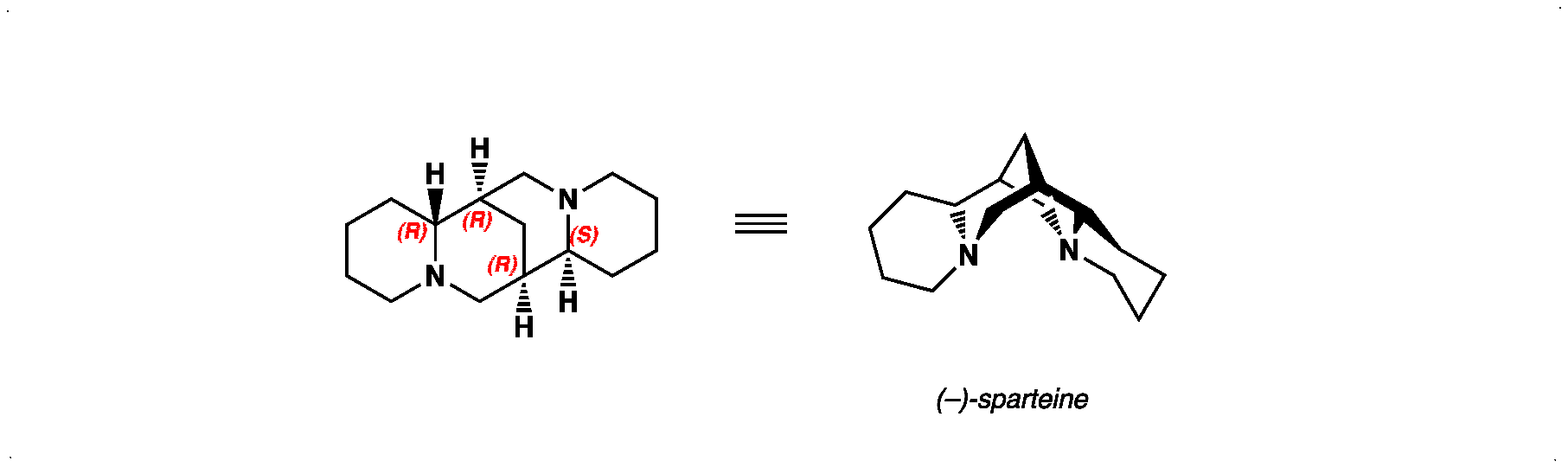
Enantioselective deprotonation of a prochiral carbon under the control of a bidentate chiral ligand offers a route to the synthesis of enantioenriched products. A 1:1 ratio of isopropyllithium (i-PrLi) and (–)-sparteine (see below for structure) can form a complex with a suitable substrate to give the desired stereodefined organolithium (Step 1). Here we give an example of such a reaction with Boc-pyrrolidine – followed by addition of the chiral nucleophile to benzophenone (Step 2).
Click the reactions below to view a 3D model of the reaction:
K. B. Wiberg and W. F. Bailey, J. Am. Chem. Soc., 2001, 123, 8231–8238.