NOTE: Important charges and non-bonding electrons are shown throughout the animation except during the transition phase
Click the structures and reaction arrows to view the 3D models and animations respectively
Cis-trans isomerisation and solvent reorganisation throughout the reaction are not shown explicitly.
For simplicity animations are shown with PH3 instead of PR3.
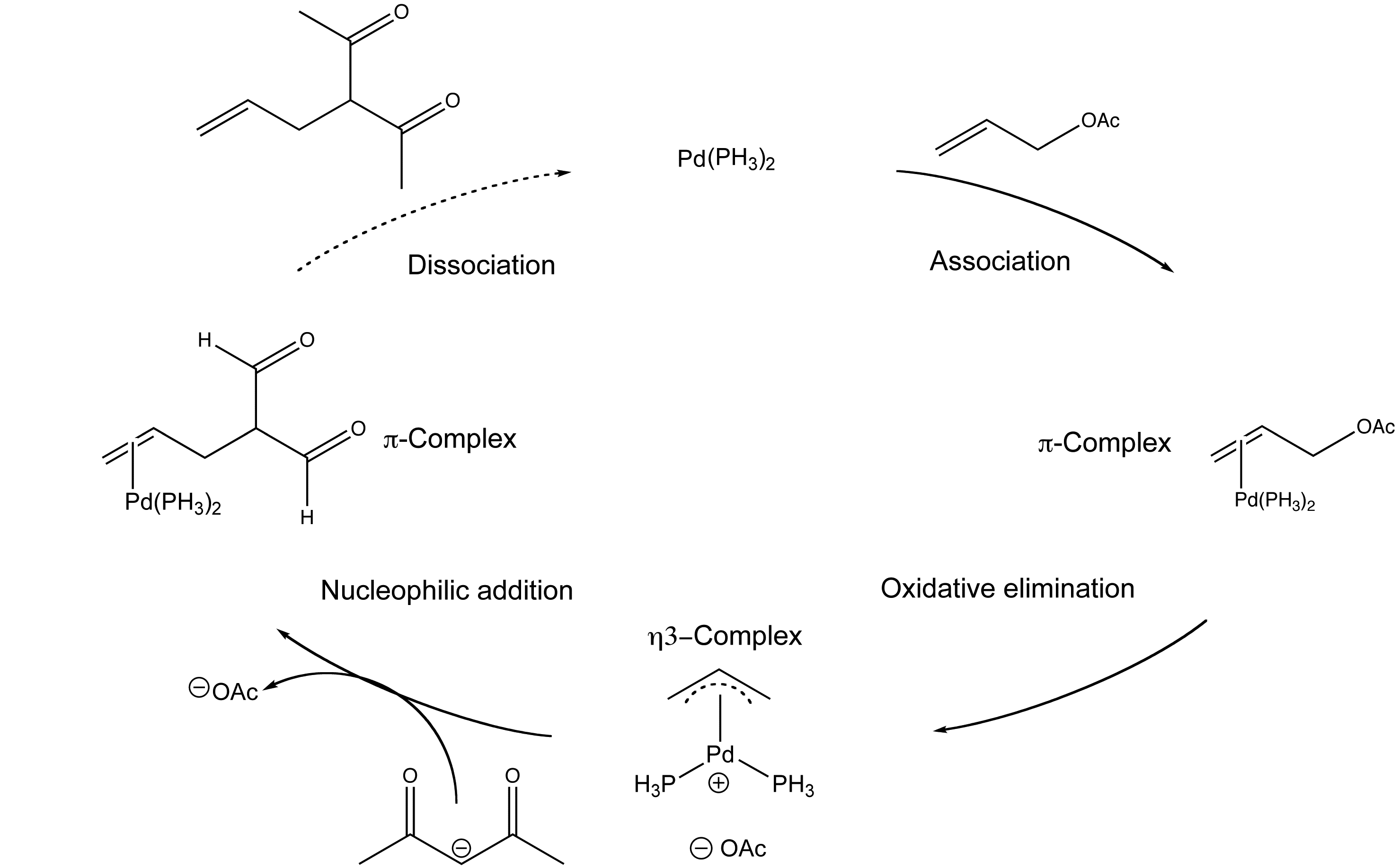
The palladium-catalysed electrophilic allylic substitution of functionalised compounds is a useful way of making C-C bonds.
The mechanism involves the association of the palladium catalyst and the allylic alkene, a oxidative addition with the newly coordinated complex, a nucleophilic addition with a malonate nucleophile and a ligand dissociation step to remove the new compound and regenerate the catalyst.
C. G. Frost, J. Howarth and J. M. J. Williams, Tetrahedron: Asymmetry, 1992, 3, 1089–1122.