Asymmetric Alkylation of Pyrrole with a MacMillan Organocatalyst
Click the structures and reaction arrows in sequence to view the 3D models and animations respectively
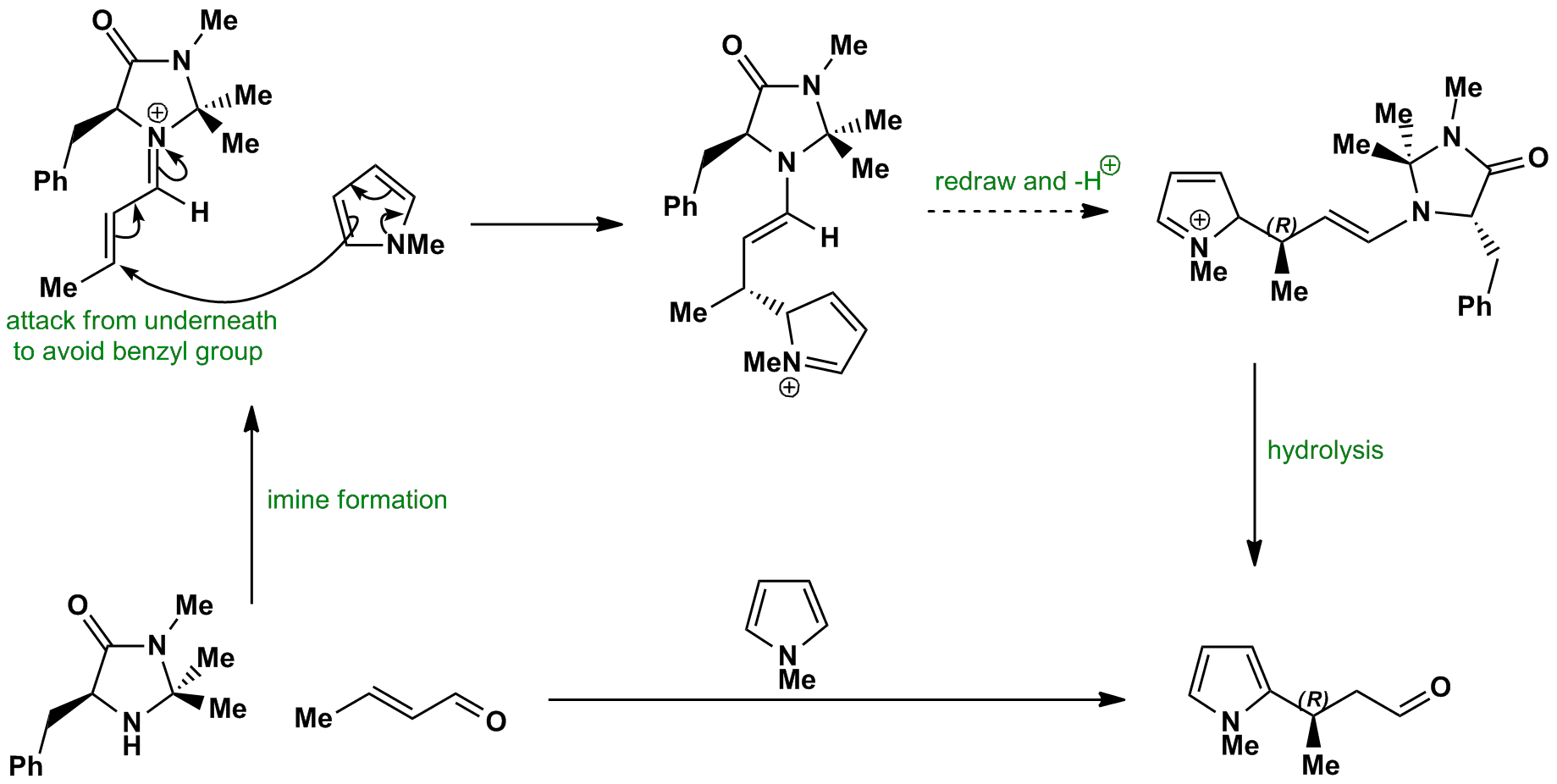
The pyrrole attacks the imine from below (to avoid the benzyl group) via a conjugate addition mechanism. The resultant enamine can then be hydrolysed to give the the alkylated pyrrole product.
Press the Substrate Spacefill On button to judge for yourself which side the nucleophile is most likely going to attack!
N. A. Paras and D. W. C. MacMillan, J. Am. Chem. Soc., 2001, 123, 4370–4371.