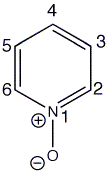
Click the checkbox to view the atom numbering
Pyridine N-oxide is formed by the oxidation of pyridine using m-CPBA or just H2O2 in acetic acid. N-oxides are stable dipolar species with the electrons on oxygen delocalised round the pyridine ring, raising the HOMO of the molecule.
Pyridine N-oxides are reactive towards both electrophilic and nucleophilic substitution at the 2-, 4- and 6- positions, this can be seen by looking at the HOMO as the lobes are a lot bigger at the 2-, 4- and 6- positions than the 3- and 5- positions.
S. Youssif, Arkivoc, 2001, 2001, 242–268.