Click the structures and reaction arrows in sequence to view the 3D models and animations respectively
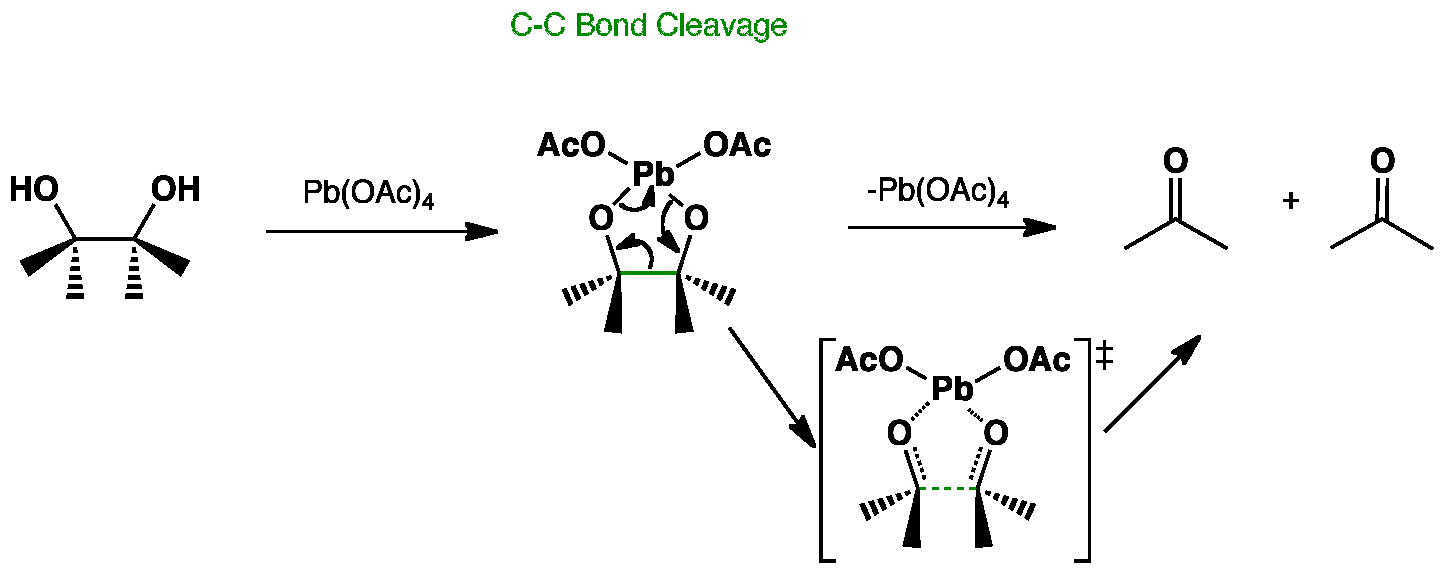
In this example lead tetraacetate is used cleave a carbon-carbon bond in a vicinal diol (glycol).
This reaction is useful in the formation of ketones and aldehydes and involves a favourable five-membered cyclic intermediate.
A.-K. Schmidt and C. Stark, Synthesis (Stuttg)., 2014, 46, 3283–3308.