<
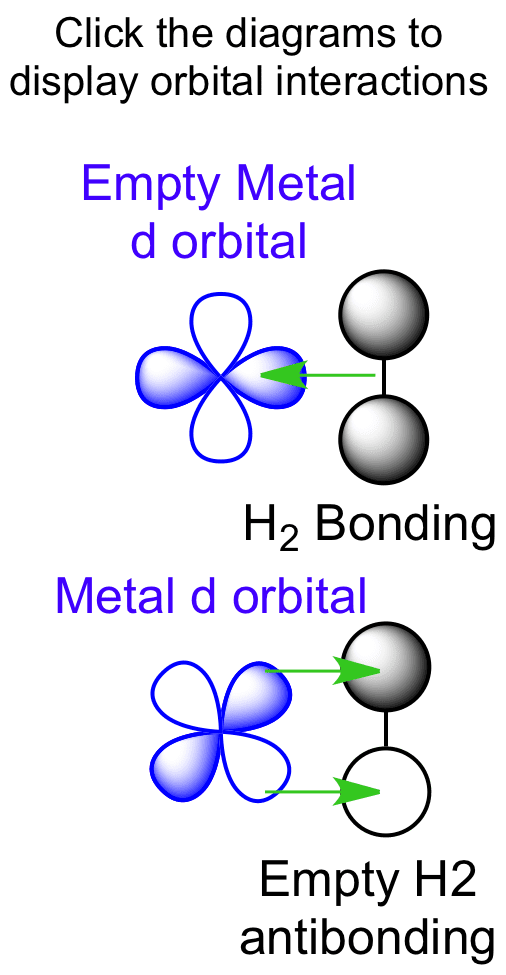
The bonding of dihydrogen involves a combination of π and σ bonding. The σ donation comes from the bonding dihydrogen orbital. The π backbonding arises from donation of metal d orbital electron density into the dihydrogen antibonding orbital.
View Hydrogen Molecular Orbitals here
Explore Metal-Ligand bonding with other molecules
Carbon Monoxide | Phosphine | Hydrogen | Ethylene | Cyclobutadiene | Butadiene | Benzene | Allyl | Cyclopentadienyl | Carbene