NOTE: Important charges and non-bonding electrons are shown throughout the animation except during the transition phase. Silver has been omitted from the 3D animation.
Click the structures and reaction arrows to view the 3D models and animations respectively
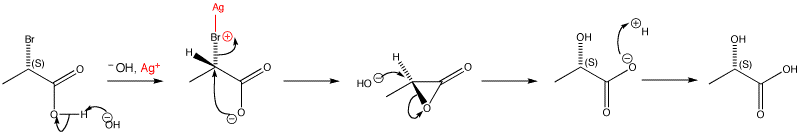
Nucleophilic substitution reactions that go with retention of stereochemistry are rather rare and most likely go through two successive inversions with neighbouring group participation. The neighbouring group is carboxylate: the silver oxide is important because it encourages the ionization of the starting material by acting as a halogen-selective Lewis acid. A three-membered ring intermediate forms, which then gets opened by hydroxide in a second SN2 step.
S. Winstein and R. E. Buckles, J. Am. Chem. Soc., 1942, 64, 2780–2786.