Use the buttons to display the 4 sp3 orbitals that result from combining one s and three p orbitals. The wireframe model is the best choice for showing all the orbitals.
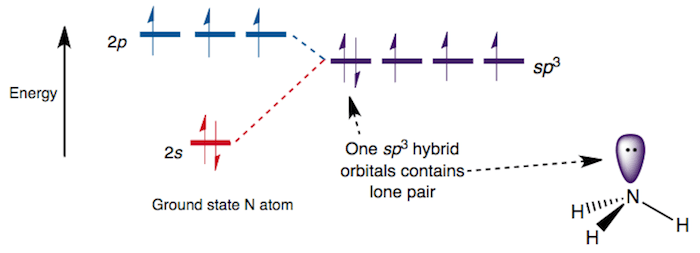
Each N–H σ-bonding orbital, containing 2 electrons, is formed from a N sp3 hybrid orbital and a H 1s orbital. The remaining lone pair of electrons occupies the fourth tetrahedral position producing a pyramidal structure.
Explore bonding orbitals in other small molecules
Hydrogen | Fluorine | Nitrogen | Hydrogen Fluoride | Carbon Monoxide | Methane | Ammonia | Ethylene | Acetylene | Allene | Formaldehyde | Benzene