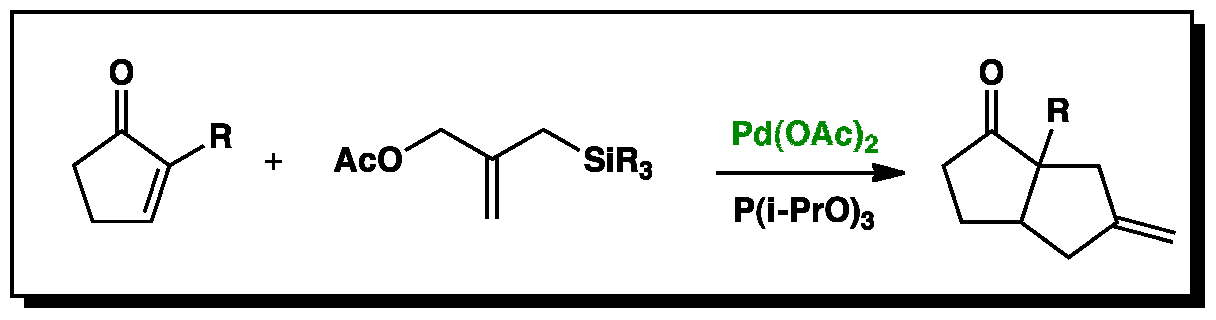
Overall Reaction
The presence of five membered ring systems in many target molecules has produced varied methods of their preparation. One of the most successful of these is the trimethylenemethane [3 +2] cycloaddition. This process is catalysed by palladium (0) complexes produced in situ by the reduction of Pd(OAc)2 by Pd(i-PrO)3. In these animations a Pd(PH3) complex has been used for modelling purposes.
Click the the different stages to view the 3D models of each reaction:
Stage 1
This stage of the reaction forms the trimethylenemethane unit necessary for the cycloaddition from a [(trimethylsilyl)methyl]-2-propen-1yl acetate.
The palladium Pi-allyl complex is formed followed by the removal of the trimethylsilyl group under nucleophilic attack from the resulting acetate ion.
Stage 2
Overall the mechanism for this reaction is proposed to be a stepwise ‘cycloaddition’ reaction, whereby the carbanion attacks the cyclopentenone first to form an enonlate. The resulting enolate can then cyclise to form exo five membered cycloaddition product.