NOTE: Important charges and non-bonding electrons are shown throughout the animation except during the transition phase
Click the structures and reaction arrows to view the 3D models and animations respectively
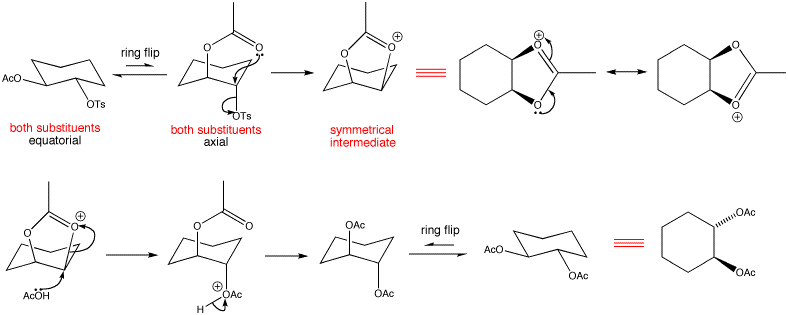
The increase in reaction rate indicates that neighbouring group particiaption is taking place. The neighbouring groups only become involved if they can increase the rate of the substitution reaction. Not much can happen when both substituents are equatorial, but if we allow the ring to flip the acetate substituent is ideally placed to participate in the departure of the tosylate group. Overall, we have retention of stereochemistry which means we have neighbouring group participation.