Click the structures and reaction arrows in sequence to view the 3D models and animations respectively
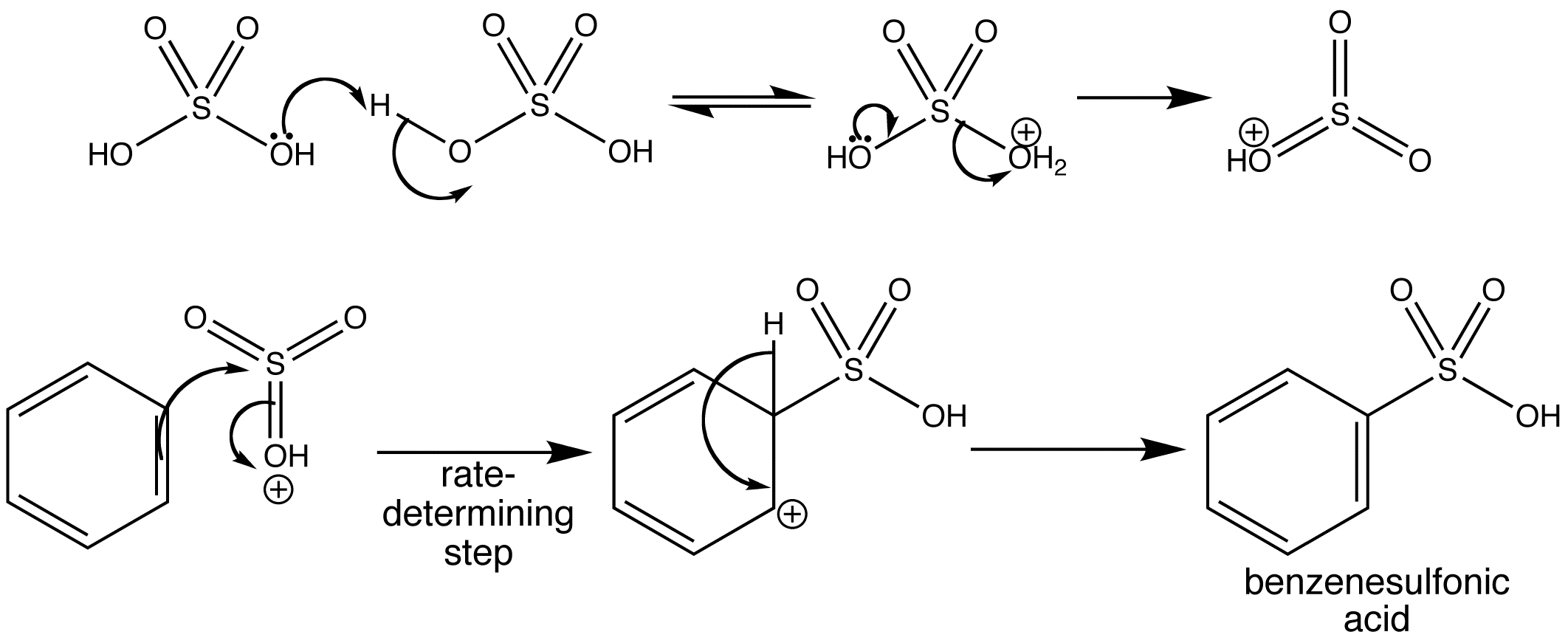
Benzene reacts slowly with sulfuric acid alone to give benzenesulfonic acid. The reaction starts with the protonation of one molecule of sulfuric acid by another, followed by the loss of a molecule of water. The cation produced is very reactive and combines with benzene via the slow addition to the aromatic π
system, followed by rapid loss of a proton to regenerate the aromaticity. The product contains the sulfonic acid functional group -SO2OH.
G. A. Olah, Acc. Chem. Res., 1971, 4, 240–248.