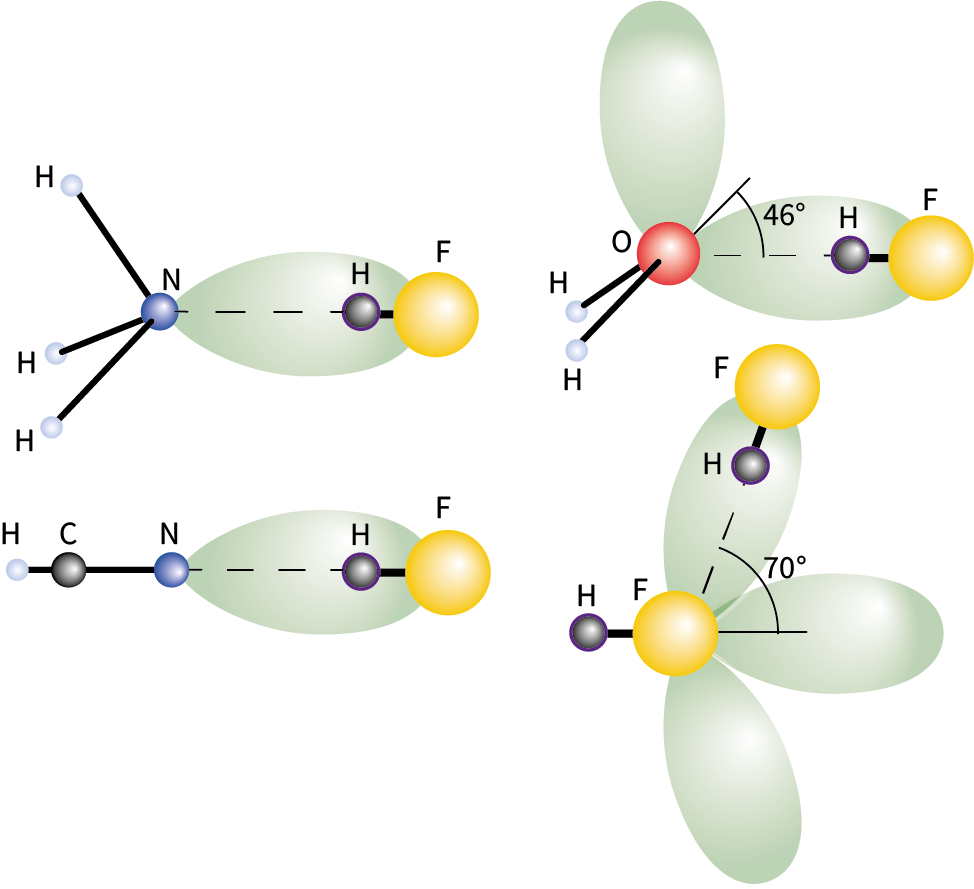
The H-bonding interactions between gaseous HF and other small molecules, showing how their lone-pairs dictate the shapes of the adducts formed. The HF molecule (shown as enlarged spheres) is oriented along the three-fold axis of NH3, collinear with HCN, out of the HOH plane in its complex with H2O, and off the HF axis in the (HF)2 dimer.