Click the structures and reaction arrows in sequence to view the 3D models and animations respectively
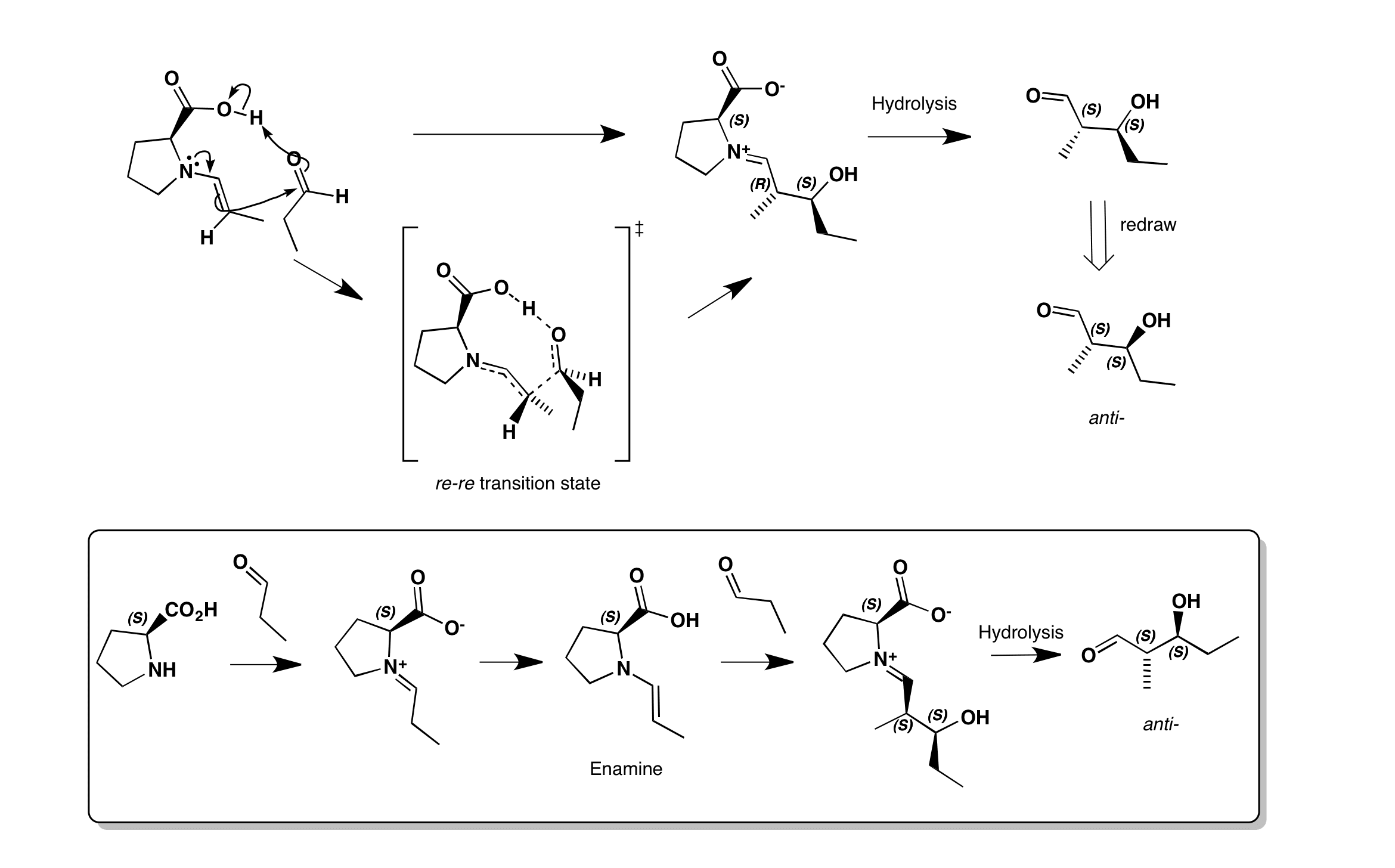
The enamine attacks propanal with the Re face onto propanal’s Re face. This causes a Re-Re transition state. This transition state produces an (S,S) iminium, which on hydrolysis forms the (S,S)product also. The (S,S)-3-hydroxy-2-methylpentanal has anti stereochemistry.
Click for more information on Re and Si.
A. K. Sharma and R. B. Sunoj, Angew. Chemie Int. Ed., 2010, 49, 6373–6377.
J. Liu and L. Wang, Synthesis (Stuttg)., 2016, 49, 960–972.