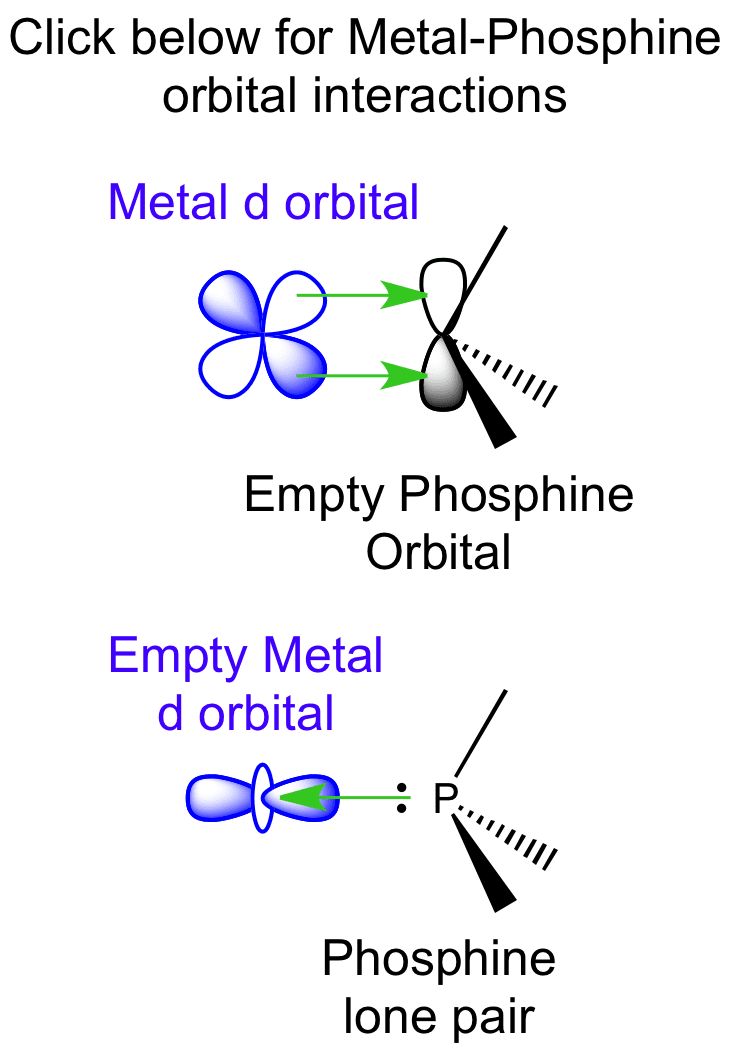
The bonding of a phosphine ligand is similar of that of carbon monoxide. Phosphine can act as a Lewis σ base by donating its electron lone pair. The empty orbitals on phosphine can accept electron density from the metal d orbitals via π backbonding. Hence phosphine can be considered a Lewis σ base and a Lewis π acid.
Explore Metal-Ligand bonding with other molecules
Carbon Monoxide | Phosphine | Hydrogen | Ethylene | Cyclobutadiene | Butadiene | Benzene | Allyl | Cyclopentadienyl | Carbene