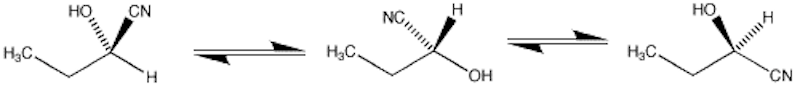
Conformation is different to configuration. Conformations differ only in the temporary way the molecule happens to arrange itself, and can easily be interconverted just by rotating around bonds. No bonds are broken.
Click the structures and reaction arrows to view the 3D models and animations respectively
The molecules above have no plane of symmetry and can exist as enantiomers.
Next page: Conformations of ethane