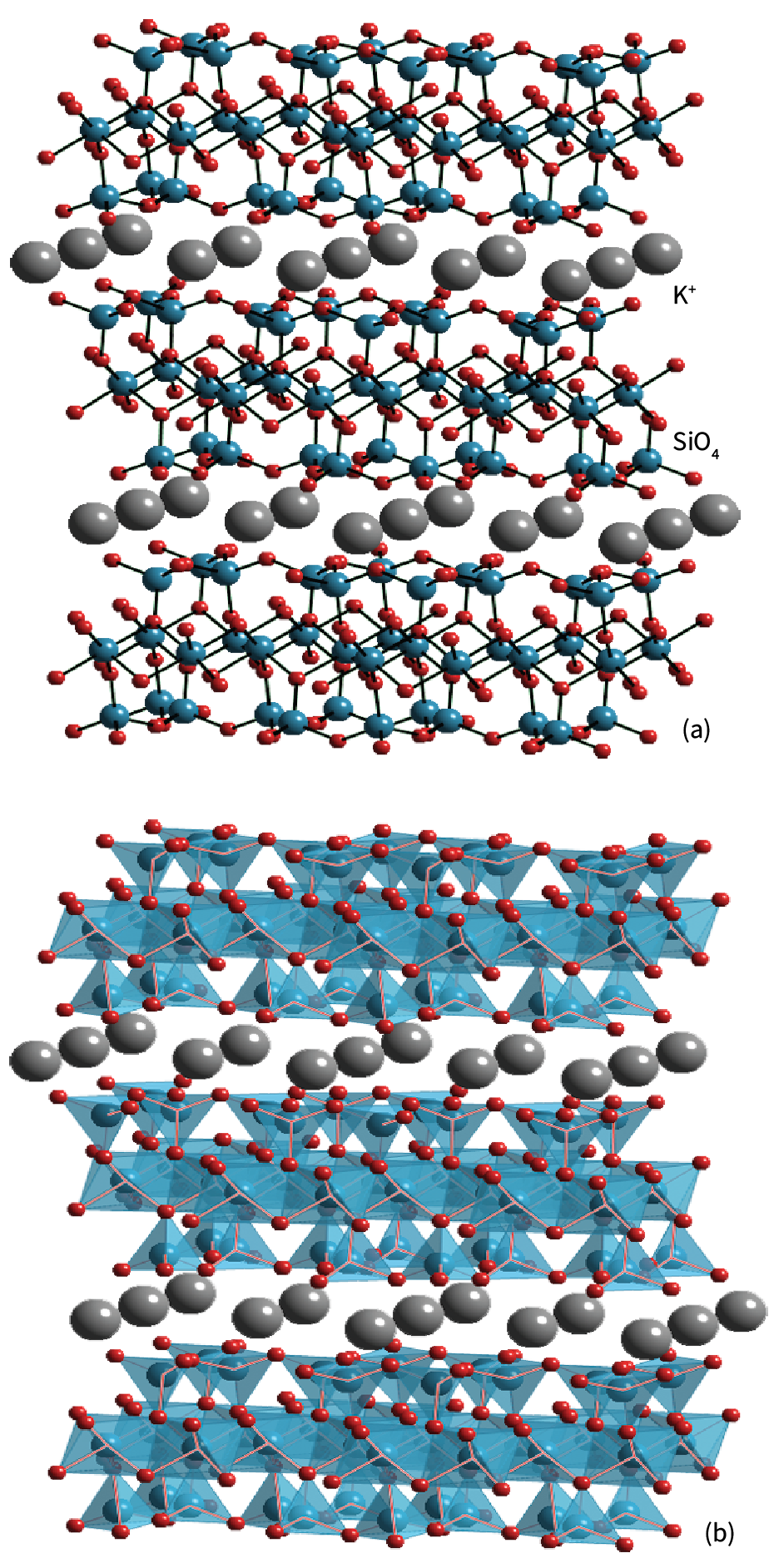
(a) The structure of 2:1 clay minerals such as muscovite mica KAl2(OH)2Si3AlO10, in which K+ resides between the charged layers (exchangeable cation sites), Si4+ resides in sites of coordination number 4, and Al3+ in sites of coordination number 6.
(b) The polyhedral representation. In talc, Mg2+ ions occupy the octahedral sites and O atoms on the top and bottom are replaced by OH groups and the K+ sites are vacant.